28
Oct
2025

Shenzhen Haorui Pharmatech Co., Ltd
Exhibitor at CPHI Frankfurt 2025 stand 4.1J2, Natural Extracts
About Us
Categories

-
CN
-
2015On CPHI since
-
100 - 249Employees
Company types
Event information
CPHI Frankfurt 2025
-
28 Oct 2025 - 30 Oct 2025
-
Messe, Frankfurt
-
Visit us at stand 4.1J2, Natural Extracts
Products Featured at CPHI Frankfurt 2025
-
Product Dasatinib anhydrous/monohydrate
We offer a wide range of API products which include Dasatinib monohydrate and Dasatinib anhydrous. It belongs to oncology category. Therapeutic category: molecular targeted. Regulatory status for Dasatinib monohydrate:USDMF No. 23763, USFDA Approved, Stability Studies Zone IVB, WC available ... -
Product Docetaxel anhydrous
We offer a wide range of API products which includes Docetaxel anhydrous. It belongs to oncology category. Therapeutic category: antineoplastic.
Other products:Amisulpride,Desvenlafaxine Succinate,Neostigmine methylsulfate
Teicoplanin,Daptomycin,Minocycline HCl,Vancomycin HCl, Levofloxacin... -
Product Irinotecan HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Irinotecan HCl. It belongs to oncology category. Therapeutic category: antineoplastic.
Regulatory status of Irinotecan HCl:USDMF No. 23522, USFDA Approved,
CEP: R0-CEP 2020-025- Rev 00
Stability... -
Product Granisetron Base/Hydrochloride
We provide high quality Granisetron with DMF.Regulatory status for Granisetron base:
USDMF No. 22089, USFDA Approved
For Granisetron hydrochloride:
USDMF No. 19365, USFDA Approved
CEP: R1-CEP 2009-341-Rev 03, UK GMP
Stability Studies Zone IVB, WC available
If you want to know more informa... -
Product Flumazenil
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Flumazenil. It belongs to API category. Therapeutic category: benzodiazepine.
Regulatory status of Flumazenil:
USDMF No. 17971, USFDA Approved
CEP: CEP 2008-121-Rev 02, UK GMP
Stability Studies ... -
Product Minocycline HCl
We offer Minocycline Hydrochloride of high quality with Eu GMP and DMF.Currently, there is a shortage of raw material in the world. However, our production capability is stable. -
Product Granisetron HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Granisetron HCl. It belongs to antiemetic category. Contact us for more information. -
Product Epinastine HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Epinastine HCl. It belongs to API category. Therapeutic category: antihistaminic. Contact us for more information. -
Product Granisetron Base
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Granisetron Base. It belongs to antiemetic category. Contact us for more information. -
Product Amisulpride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Amisulpride. It belongs to API category. Therapeutic category: antipsychotic. Contact us for more information. -
Product Abacavir sulfate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Abacavir sulfate. It belongs to API category. Therapeutic category: antiviral. Contact us for more information. -
Product SN-38
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes SN-38. It belongs to oncology category. Therapeutic category: antineoplastic. Contact us for more information. -
Product 9-Aminominocycline sulfate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 9-Aminominocycline sulfate. It belongs to intermediates category. Therapeutic category: tigecycline. Contact us for more information. -
Product Pregabalin
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Pregabalin. It belongs to API category. Therapeutic category: anticonvulsant. Contact us for more information. -
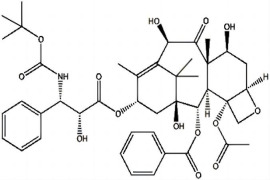
Product Paclitaxel
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Paclitaxel. It belongs to oncology category. Therapeutic category: antineoplastic. Contact us for more information. -
Product Alprostadil
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Alprostadil. It belongs to prostaglandin category. Therapeutic category: vasodilator. Contact us for more information. -
Product Daptomycin
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Daptomycin. It belongs to anti-infection category. Therapeutic category: antibacterial. Contact us for more information. -
Product Fipronil
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Fipronil. It belongs to veterinary category. Therapeutic category: ectoparasiticide. Contact us for more information. -
Product (+/-)-3-Amino-9,13b-dihydro-1H-dibenz[c,f]imidazo[1,5-a]azepine hydrobromide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (+/-)-3-Amino-9,13b-dihydro-1H-dibenz[c,f]imidazo[1,5-a]azepine hydrobromide. It belongs to intermediates category. Therapeutic category: epinastine. Contact us for more information. -
Product (-)-Corey lactone benzoate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (-)-Corey lactone benzoate. It belongs to intermediates category. Therapeutic category: prostaglandin. Contact us for more information. -
Product (-)-Corey lactone 4-phenylbenzoate alcohol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (-)-Corey lactone 4-phenylbenzoate alcohol. It belongs to intermediates category. Therapeutic category: prostaglandin. Contact us for more information. -
Product (+)-Corey lactone diol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (+)-Corey lactone diol. It belongs to intermediates category. Therapeutic category: entecavir. Contact us for more information. -
Product (-)-Corey lactone diol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (-)-Corey lactone diol. It belongs to intermediates category. Therapeutic category: prostaglandin. Contact us for more information. -
Product (1R,2R)-1,2-Cyclohexanedimethanol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (1R,2R)-1,2-Cyclohexanedimethanol. It belongs to intermediates category. Therapeutic category: lurasidone. Contact us for more information. -
Product (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium(2R)-hydroxy (phenyl)ethanoate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium(2R)-hydroxy (phenyl)ethanoate. It belongs to intermediates category. Therapeutic category: ticagrelor. Contact us for more information. -
Product (1R,2S)-2-Fluorocyclopropylamine tosylate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (1R,2S)-2-Fluorocyclopropylamine tosylate. It belongs to intermediates category. Therapeutic category: sitafloxacin. Contact us for more information. -
Product (6S)-5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (6S)-5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one. It belongs to intermediates category. Therapeutic category: dorzolamide. Contact us for more information. -
Product (1R,2R)-1,2-Cyclohexanedicarboxylic acid
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (1R,2R)-1,2-Cyclohexanedicarboxylic acid. It belongs to intermediates category. Therapeutic category: lurasidone. Contact us for more information. -
Product (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol. It belongs to intermediates category. Therapeutic category: ticagrelor. Contact us for more information. -
Product (3S,5S)-3-(1-Methylethyl)-2-oxo-5-[(2S,4S)-tetrahydro-4-(1-methylethyl)-5-oxo-2-furanyl]-1-pyrrolidinecarboxylic acid tert-butyl ester
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (3S,5S)-3-(1-Methylethyl)-2-oxo-5-[(2S,4S)-tetrahydro-4-(1-methylethyl)-5-oxo-2-furanyl]-1-pyrrolidinecarboxylic acid tert-butyl ester. It belongs to intermediates category. Therapeutic category: aliskiren. Contact us ... -
Product (4R,6S)-5,6-Dihydro-4-hydroxy-6-methylthieno[2,3-b]thiopyran-7,7-dioxide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (4R,6S)-5,6-Dihydro-4-hydroxy-6-methylthieno[2,3-b]thiopyran-7,7-dioxide. It belongs to intermediates category. Therapeutic category: dorzolamide. Contact us for more information. -
Product (7S-3,4-Dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7- methanamine hydrochloride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (7S-3,4-Dimethoxy-N-methylbicyclo[4.2.0]octa-1,3,5-triene-7- methanamine hydrochloride. It belongs to intermediates category. Therapeutic category: lvabradine. Contact us for more information. -
Product (R,R)-1,2-Bis(methanesulfonyloxymethyl)cyclohexane
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (R,R)-1,2-Bis(methanesulfonyloxymethyl)cyclohexane. It belongs to intermediates category. Therapeutic category: lurasidone. Contact us for more information. -
Product (R)-2-Chloropropionyl chloride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (R)-2-Chloropropionyl chloride. It belongs to intermediates category. Therapeutic category: l-alanyl-l-glutamine. Contact us for more information. -
Product (1R,4S)-4-Aminocyclopentene-1-methanol hydrochloride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (1R,4S)-4-Aminocyclopentene-1-methanol hydrochloride. It belongs to intermediates category. Therapeutic category: abacavir. Contact us for more information. -
Product (s,s)-2,8-diazabicyclo[4,3,0]nonane
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (s,s)-2,8-diazabicyclo[4,3,0]nonane. It belongs to intermediates category. Therapeutic category: moxifloxacin. Contact us for more information. -
Product (S)-3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes (S)-3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt. It belongs to intermediates category. Therapeutic category: repaglinide. Contact us for more information. -
Product [(7S)-5-(Phenylmethyl)-5-azaspiro[2.4]hept-7-yl]carbamic acid tert-butyl ester
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes [(7S)-5-(Phenylmethyl)-5-azaspiro[2.4]hept-7-yl]carbamic acid tert-butyl ester. It belongs to intermediates category. Therapeutic category: sitafloxacin. Contact us for more information. -
Product 1-(4-Amino-2-methylbenzoyl)-7-chloro-1,2,3,4-tetrahydro-5H-1- benzazepin-5-one
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 1-(4-Amino-2-methylbenzoyl)-7-chloro-1,2,3,4-tetrahydro-5H-1- benzazepin-5-one. It belongs to intermediates category. Therapeutic category: tolvaptan. Contact us for more information. -
Product 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8- bromoxanthine
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8- bromoxanthine. It belongs to intermediates category. Therapeutic category: linagliptin. Contact us for more information. -
Product 1-Deoxynojirimycin
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 1-Deoxynojirimycin. It belongs to plant extract category. Therapeutic category: protection. Contact us for more information. -
Product 1-Methylhexahydroazepin-4-one hydrochloride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 1-Methylhexahydroazepin-4-one hydrochloride. It belongs to intermediates category. Therapeutic category: azelastine. Contact us for more information. -
Product 2-((4-Chlorophenyl)acetyl)benzoic acid
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-((4-Chlorophenyl)acetyl)benzoic acid. It belongs to intermediates category. Therapeutic category: azelastine. Contact us for more information. -
Product 2-(5-Mercaptotetrazole-1-yl)ethanol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-(5-Mercaptotetrazole-1-yl)ethanol. It belongs to intermediates category. Therapeutic category: flomoxef. Contact us for more information. -
Product 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H- cyclopenta-1,3-dioxol-4-yl]oxy]ethanol
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H- cyclopenta-1,3-dioxol-4-yl]oxy]ethanol. It belongs to intermediates category. Therapeutic category: ticagrelor. Contact us for more information. -
Product 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-ethanol (2R,3R)-2,3-dihydroxybutanedioate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-ethanol (2R,3R)-2,3-dihydroxybutanedioate. It belongs to intermediates category. Therapeutic category: ticagrelor. Contact us for mo... -
Product 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl bromide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl bromide. It belongs to intermediates category. Therapeutic category: silodosin. Contact us for more information. -
Product 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate. It belongs to intermediates category. Therapeutic category: silodosin. Contact us for more information. -
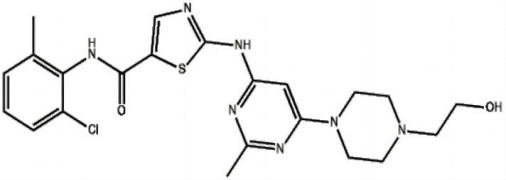
Product 2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 2-Amino-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide. It belongs to intermediates category. Therapeutic category: dasatinib. Contact us for more information. -
Product 28-Homobrassinolide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 28-Homobrassinolide. It belongs to API category. Therapeutic category: bio-pesticide. Contact us for more information. -
Product 24-Epibrassinolide
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 24-Epibrassinolide. It belongs to API category. Therapeutic category: bio-pesticide. Contact us for more information. -
Product 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one. It belongs to intermediates category. Therapeutic category: lvabradine. Contact us for more information.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance