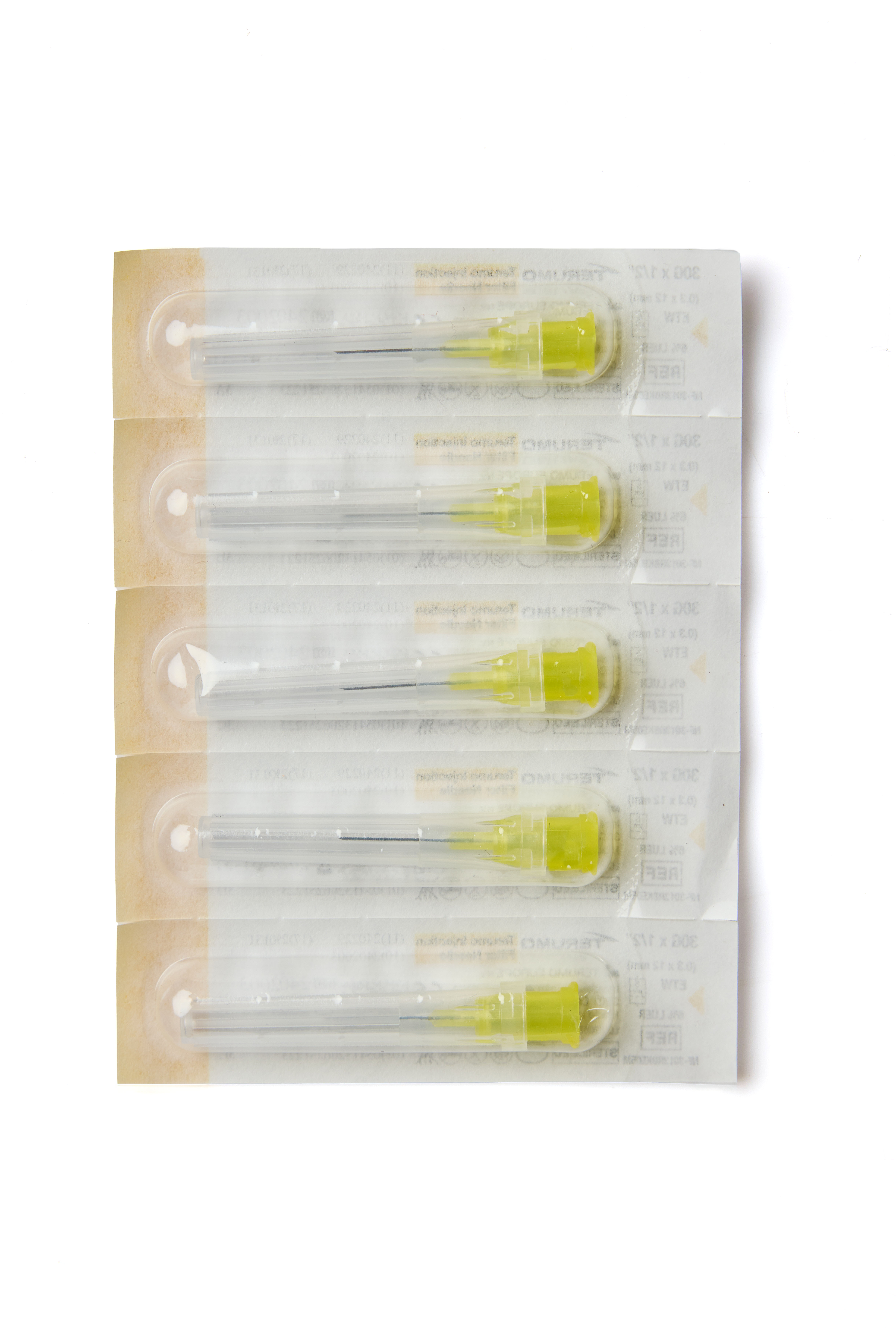
INFINO™
Product Description
Terumo Europe NV

-
BE
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Terumo Europe NV

-
BE
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Terumo Europe NV (11)
-
Product SurGuard™2 Safety Hypodermic Needles
The SurGuard™2 needle has been developed to meet the market’s requirements and offers you a simple SAFETY solution.
It's simple locking mechanism will reduce needle stick injuries significantly and can easily be activated by using only one hand!
The SurGuard™2 needle’s standard hub eq... -
Product K-Pack™ Surshield™ Needles with passive sharps protection
K-Pack™ Surshield™ needles are hypodermic needles equipped with a passive sharps protection intended to offer needlestick prevention solutions that support therapeutic success. -
Product Surflo™ Winged Infusion Set with/without Needle Protection
Surflo™ Winged Infusion Sets with disposable infusion apparatus, are used for intravenous injection.
Surflo™ Winged Infusion Sets with needle protection (Surshield™) minimize the risk of accidental needle stick. Once activated, the needle is perman... -
Product NEOLUS™ Needles
NEOLUS™ needles are sterile, single-use hypodermic needles, intended to inject fluids into, or withdraw fluids fromparts of the body below the surface of the skin. -
Product Nanopass™ 32.5G Pen Needle
Our Nanopass™ 32.5G Pen Needle for Pen Injector offers a reduced diameter thin-wall needle and unique asymmetric lance bevel. -
Product Nanopass™ 34G Pen Needle
Nanopass™ 34G Needle for Pen Injector features a 22% thinner double-tapered needle aiming at a better flow than comparable 32G designs. -
Product K-Pack™ II Needles
K-Pack™ II Needles are carefully crafted to achieve smoother injections of today and tomorrow’s parenteral medications – targeting on a less painful injection and thus on higher therapeutic adherence and compliance. -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Tapered Needle
PLAJEX™ Ready-to-Fill Polymer Syringes with Tapered Needles offer the better injectability without compromising patient comfort for pharmaceutical manufacturers who need to overcome injectability problems associated with highly viscous concentrated formulations.
(Assessment of the Injection Performa... -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Luer Lock
PLAJEX™ Ready-to-Fill Polymer Syringes with Luer Lock offerthe quality and supply reliability needed to meet your parenteral delivery challenges. -
Product SurGuard™3 Safety Hypodermic Needles
SurGuard™3 Safety Hypodermic Needles featured by Terumo Medical Care Solutions Pharmaceutical Division are equipped with a three-way needlestick protection system and utilize Terumo’s T-Sharp™ technology – offering Terumo quality needles. -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Staked Needle
PLAJEX™ Ready-to-Fill Polymer Syringes with Staked Needles provide lower interactions with proteins for pharmaceutical manufacturers who need to deliver today’s most challenging biopharmaceuticals.
(Functional Evaluation and Characterization of a Newly Developed Silicone Oil-Free Prefillable Syr...
Terumo Europe NV resources (6)
-
News Pharmapack 2025 Track Sponsor Interview: Terumo on the importance of packaging innovation
At Pharmapack 2025 in Paris in January, the conference theatre will once again be hosting insightful discussions across several key topics in the pharmaceutical packaging field. One track, Device and Packaging Innovation will be sponsored by Terumo. -
Video Terumo Medical Care Solutions - CDMO
Access world-class CDMO capabilities.
We provide sterile injectable CDMO from clinical to commercial scale. Drawing on 100 years of expertise, our integrated services include combination product design, formulation development, aseptic fill & finish, device assembly and packaging. -
News Pharmapack 2023: Session interview – Terumo on integrated CDMO solutions
Pharmapack 2023 showcased a number of sessions on the key topics in the industry, from conference sessions to workshops, presented by experts in the field. -
Video Pharmaceutical Solutions Division in video
Discover who we are and how we work. Get a glimpse of our state of the art production. -
News CPHI Frankfurt 2022: Innovator Interview – Terumo
In this series of interviews, we speak to the companies on the CPHI Frankfurt show floor who are driving innovation in pharma for a better healthcare future. We chatted to Marco Chiadò Piat (President) and Anil Busimi (VP Strategy and Marketing) of Terumo Pharmaceutical Solutions about how events such as CPHI can help all companies across the pharmaceutical supply chain. -
Video PLAJEX™ by Terumo
PLAJEX™ Terumo's pre-fillable COP syringes
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance