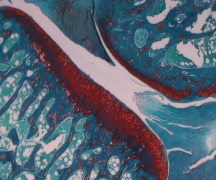
Food safety evaluation
Product Description
galileo research

-
IT
-
2023On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
galileo research

-
IT
-
2023On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
Primary activities
More Products from galileo research (4)
-
Product Biocompatibility of Medical Devices according to ISO 10993-1 series
Galileo Research can drive the nonclinical development of your medical device by preparing the Literature Report and the Biological Evaluation Plan (BEP) according to ISO 10993-1, performing additional tests, if needed, and releasing the Biological Evaluation Report (BER).
All tests can be condu... -
Product Consultancy for drug/MD/food development
Our expertise offered in regulatory submissions and knowledgeable strategic skills in planning non-clinical testing programmes can reduce attrition rate and speed up the process.
-
Product Drug efficacy, In vitro and in vivo studies
We offer a full range of studies and bioanalytical services, providing powerful tools and rigorous solutions for preclinical drug development of therapeutic candidates, advance lead compounds towards first-in- human trials, and support for ongoing clinical development -
Product Drug safety, in vitro and In vivo studies
In support of human clinical trials and marketing authorization approval, Galileo Research offers a full battery of toxicity studies for the regulatory development of small drugs, as well as biologics and advanced therapies, in compliance with Good Laboratory Practice (GLP), ICH and OECD guidelines.
galileo research resources (2)
-
Brochure Galileo Research brochure
Brief description of the company profile and the main services offered by Galileo Research. -
Brochure Galileo-Research-brochure-PDF.pdf
Brief description of the company profile and the main services offered by Galileo Research.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













































.jpg)