AmpleLogic Electronic Log Book is a web-based application specifically deals with the requirements related to the Calibration, Equipment Usage, Cleaning, Stability Schedule, Standards Usage, Chemical Usage Logs, and other details log for all equipment’s. This eLog book application converts these manual paper forms into electronic versions and ensures all the entered details or logs are completed and recorded at the right time which can be verified, reviewed and approved through approval workflows
This eLog Book/Electronic Log Book provides transparency and information sharing across all key operational departments such as quality, production, planning, and maintenance bringing visibility of production and QC lab operations to the entire organization
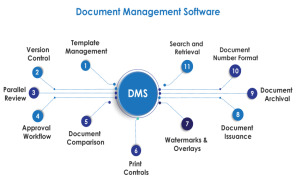
Key Features
- Automatic notification, escalations to ensure that individual logs like equipment logs, calibration logs, HPLC Column Logs, maintenance logs, cleaning, stability schedule logs, chemical usage logs, quality observation logs, training attendance logs, and other instrument logs are up to date and have been verified and reviewed on time
- Maintain Calibration, Maintenance Service Logs, Equipment Cleaning Logs, Packing Material Logs, Out of calibration investigation logs, OOS Investigation Extension and Equipment calibration logs, etc.
- Maintain SOP numbering register, quality observations, SOP issuance register for uncontrolled copies, master SOP index and request issue and retrieval of bound books
- Maintain other logs details of boiler operation daily log sheet, differential pressure monitoring record, environment condition record for manufacturing rooms, etc.
- Maintain Area and Equipment cleaning, usage logs of Standards, Columns, Chemical, Stability Schedules, ANDA and DMF Tracking Logs, etc.
- User groups or departmental users will be alerted for expired usage logs
- Workflow can be paired with any type of review and approval requirements with required electronic signatures as per the approved workflow procedures.
- eLog Book solution is compliant with FDA requirements for CFR Title 21 including 21 CFR Part 11 that outlines electronic records and signatures