Document Management System
.png)
Product Description
AmpleLogic

-
IN
-
2018On CPHI since
Categories
Specifications
AmpleLogic

-
IN
-
2018On CPHI since
More Products from AmpleLogic (2)
-
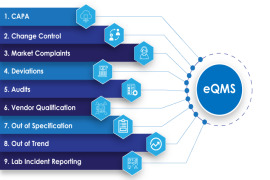
Product AmpleLogic Quality Management System Software
AmpleLogic QMS Software automates quality processes like CAPA, Change Control, Deviation, OOS, OOT, Audit Management, Vendor Qualification in pharma and life-science manufacturing companies who do it manually. All the AmpleLogic modules are 21 CFR Part 11 and EU annexure 11 compliant. Automating QMS helps ... -
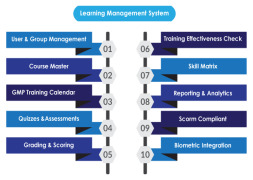
Product Learning Management System
AmpleLogic GMP Learning Management software is built to manage pharma specific employee training and Training life cycle starting from Induction till employee’s total tenure in the organisation as per 21 CFR Part 11, EU ANNEX, GMP and other regulatory requirements. AmpleLogic Biochemical Learnin...
AmpleLogic resources (1)
-
Brochure AmpleLogic GMP Compliance Software for Quality Operations
The brochure explains in brief about AmpleLogic, list of products, list of customers, implementation methodology followed and why AmpleLogic companies should choose AmpleLogic for their Quality Process Automation.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance