Annual Product Quality Review (APQR) will be conducted for each commercial product manufactured in the Pharmaceutical, Biotech and Bio-similar Industry. The aim of this annual product review within the pharmaceutical companies is to verify the consistency of the process, to assess trend, to determine the need for the changes in the specifications, production, manufacturing, Standard operating procedures(SOP) and also to evaluate the need for re-validation.
Annual Product Quality Review (APQR) in pharmaceutical and biotech companies are important for communication between manufacturing, quality, and regulatory affairs, to enable quality improvement process. Content and management of Annual Product Reviews should be established according to the regulatory requirements.
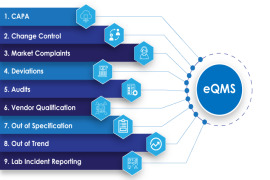
AmpleLogic APQR Software Captures the information manually and also extract the data by integrating with other software systems namely LMS, QMS, BMS (Building Management Systems), MES/ eBMR and historian databases which are connected with equipment.
Key information captured in APR Software :
- Batch details that include Product Code, Shelf life, Manufacturing, and Expiry dates. Pack Size and Process wise Yield details like Granulation, Compression, Coating, and Packing
- Raw material, packaging materials
- Process control and Analytical test results
- Batch failure or Batch Rejections
- Equipment and utilities utilized during the making of the Product and their Qualification Status e.g. HVAC, Water, Compressed Gas, etc.
- Out of specification and out of trend results
- In Process Parameters for Granulation, Compression, Coating and Packing Processes
- Product returns, Market complaints, Product recalls
- Product Stability information month and condition wise
- Analytical Method Validation and revalidation data
- Microbiological method Validation and revalidation data
- Generating Trends Batch wise on Granulation Yield, Compression Yield, Coating Yield, and Packing Yield.
- Create dashboards for trends of each in-process parameter captures in each or every process along with a Lower limit and Upper limit