26
Nov
2024

Oasis Test House (AHM) LLP
Exhibitor at CPHI & PMEC India 2024 stand 15.A57
About Us
Categories

-
IN
-
2018On CPHI since
Event information
CPHI & PMEC India 2024
-
26 Nov 2024 - 28 Nov 2024
-
India Expo Centre, Greater Noida, Delhi NCR
-
Visit us at stand 15.A57
Products Featured at CPHI & PMEC India 2024
-
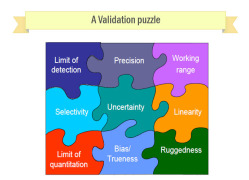
Product Method Development and Validation services
Method development and validation can be a complex story, and Oasis Test House has systematic procedures to guide its Customers through the process. Our team team of dedicated and qualified personnel are competent to develop methods and validate them as per ICH Guidelines. Before we begin a ... -
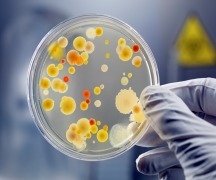
Product Microbiological Analysis
Oasis Test House caters to the microbiological requirements for its clients serving them with the below analysis. • Preservative Efficacy Analysis • Sterility Test • Sterility Validation • Microbial Limit Test Validation • Microbial count and pathogens • Bioburden testing • Antibiotics assay • Vitam... -
Product Stabiity Storage and Analysis
Stability studies for drug substances and products as per ICH Guidelines • Under investigation samples • Under approval samples • Developmental / market / production samples Assistance in protocol design, on-going sample testing and trend analysis during and at conclusion of stability testing
• Acc...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance