22
Jan
2025
Vetter Pharma-Fertigung GmbH & Co. KG
Exhibitor at Pharmapack Europe 2025 stand H37
About Us
Categories

-
DE
-
2015On CPHI since
-
3Certificates
-
5000+Employees
Company types
Primary activities
Event information
Pharmapack Europe 2025
-
22 Jan 2025 - 23 Jan 2025
-
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
-
Visit us at stand H37
Products Featured at Pharmapack Europe 2025
-
Product Drug Product Development
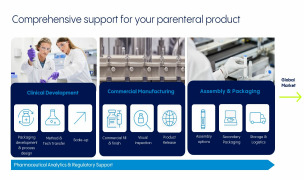
Our Vetter Development Services help biopharma customers lay a foundation of scalability, quality, and sustainable value for drug product profiles. We support drug developers in navigating key steps, decisions, and transitions in the product development cycle. This includes primary packaging evaluation, pr... -
Product Aseptic filling and visual inspection
For over 40 years, our fill and finish services have helped to keep the world supplied with injectable therapies, many of which are lifesaving. Our partnerships with customers start in the first steps of clinical manufacturing through commercial-scale filling and beyond, including management throughout the... -
Product Device Assemply and Packaging
The injectable drug market is rapidly evolving and includes an array of delivery devices from autoinjectors to pens and other intuitive formats. We provide partnership for drug owners driven by the top-quality, complex considerations and flexibility expected. These services include assembly process des... -
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com... -
Product Corporate Service Portfolio
We place heightened focus on your success as your strategic partner.
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 70+ years of history. We do not manufactu...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance