19
Jun
2024
Zhejiang Langhua Pharmaceutical Co., Ltd.
Exhibitor at CPHI & PMEC China 2024 stand W2B02b, Pharma Ingredients
About Us
Categories

-
CN
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Event information
CPHI & PMEC China 2024
-
19 June 2024 - 21 June 2024
-
Shanghai New International Expo Center
-
Visit us at stand W2B02b, Pharma Ingredients
Products Featured at CPHI & PMEC China 2024
-
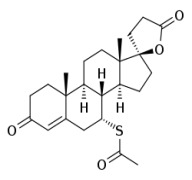
Product Spironolactone
cardiovascular system drugs CEP and USDMF registered -
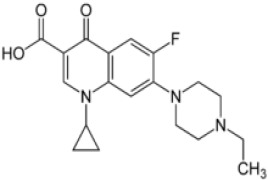
Product Enrofloxacin
Third generation Quinolones antibacterial products, veterinary use CEP and USDMF registered -
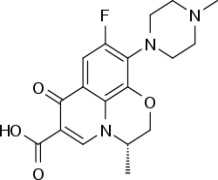
Product Levofloxacin Hemihydrate
Quinolones antibacterial product, anti-infective CEP , USDMF registered -
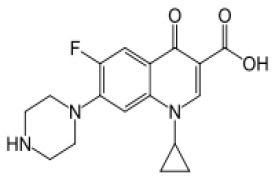
Product Ciprofloxacin Hydrochloride
Wide-range antibacterial -
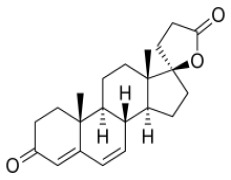
Product Canrenone
Intermediate grade: Assay: NLT 90%; NLT 97%.
API grade
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance