UMASTAR - The Best technology for dry eye relief - Preservative Free Ophthalmic Solution

Product Description
D.M.G. ITALIA S.R.L.

-
IT
-
2020On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
D.M.G. ITALIA S.R.L.

-
IT
-
2020On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from D.M.G. ITALIA S.R.L. (55)
-
Product NEO EDEMALT - Quick action against Edema - Antiedema Ointment
For a complete and rapid absorption of the edema of inflammatory and traumatic origin, reducing the related pain, swelling after sprains, bruises, sprains, muscle pulls, bumps, insect stings, post IM injections.
Indications.Medical Device with osmotic action used for treatment, in adults an... -
Product PROCTOLENIL - A quick relief from a painful Ailment - Proctologic Cream
Indications.Medication indicated in the treatment of patient suffering from hemorrhoidal syndrome, anal fissures, and proctitis. Constant use, by protecting the area of application, fosters re-epithelization and helps reducesymptoms derived from phlogosis or erythemas of any origin – including infective, m... -
Product OFTASSIALE - Corneal injuries: the respons to pain and discomfort - Preservative Free Ophthalmic Solution
Corneal pain relief, neuro-reparation, photoprotection, reepithelization.
Indications.
Corneal lubrication and protection for the treatment of eye discomfort or painful symptoms caused by foreignbodies, external agents, surgery, or traumas that may benefit from local treatment. Adjuvant the re-ep... -
Product VISCOBLAST - Dry eye and post surgical application - Carboxymethyl β-Glucan Ophthalmic Solution
Treatment of eye dryness caused by various factors.
Indications.Moisturizing, lubricating ophthalmic solution useful for the treatment of eye dryness caused by various factors(menopause, diabetes, etc…); helps the re-epithelization process; indicated for the relief of corneal hypersensitivitydue... -
Product IDROLUX - Protecting eyes from solar radiation - Photoprotective Lubricant Ophthalmic Solution
Lubricating, moisturizing, antioxidant with photoprotective action.
Indications.
Medical Device indicated in eye strain conditions caused by exposure to light sources such solar radiations(UVA/UVB/UVC, VIS, IR) or after laser refractive surgery.
General practioctioners & Specialist... -
Product OPTOREPAIR - Supporting the eye in strain conditions - Lubricant Moisturing Antioxidant Ophthalmic Solution
Lubricating, moisturizing, antioxidant with photoprotective action in eye strain conditions or after laser refractive surgery.
Indications.The oxidative stress indicates a group of reactionswhich take place in cells, in tissues and in biologicalmacromolecules when there is an excessive exposuret... -
Product CALMAHYAL - A low cost Hyaluronic Acid for all markets - Lubricant Ophthalmic Solution
Hyaluronic acid for the relief of dry eye
Indications.Lubricating ophthalmic solution indicated for the symptomatic treatment of dry eye syndrome. Also indicated forcorneal protection as a tear substitute.
General practioctioners & Specialists
Medical Device class IIb q... -
Product VITREOSAN - Vitreous well-being - Effervescent Tablets
Reintegration of useful substances to vitreous well-being.
In case of reduced dietary intake or increased need for the substances present in the product.
General practioctioners & Specialists
Nutritional Supplement
Presentation: 2 Tubes of 20 Effervescen... -
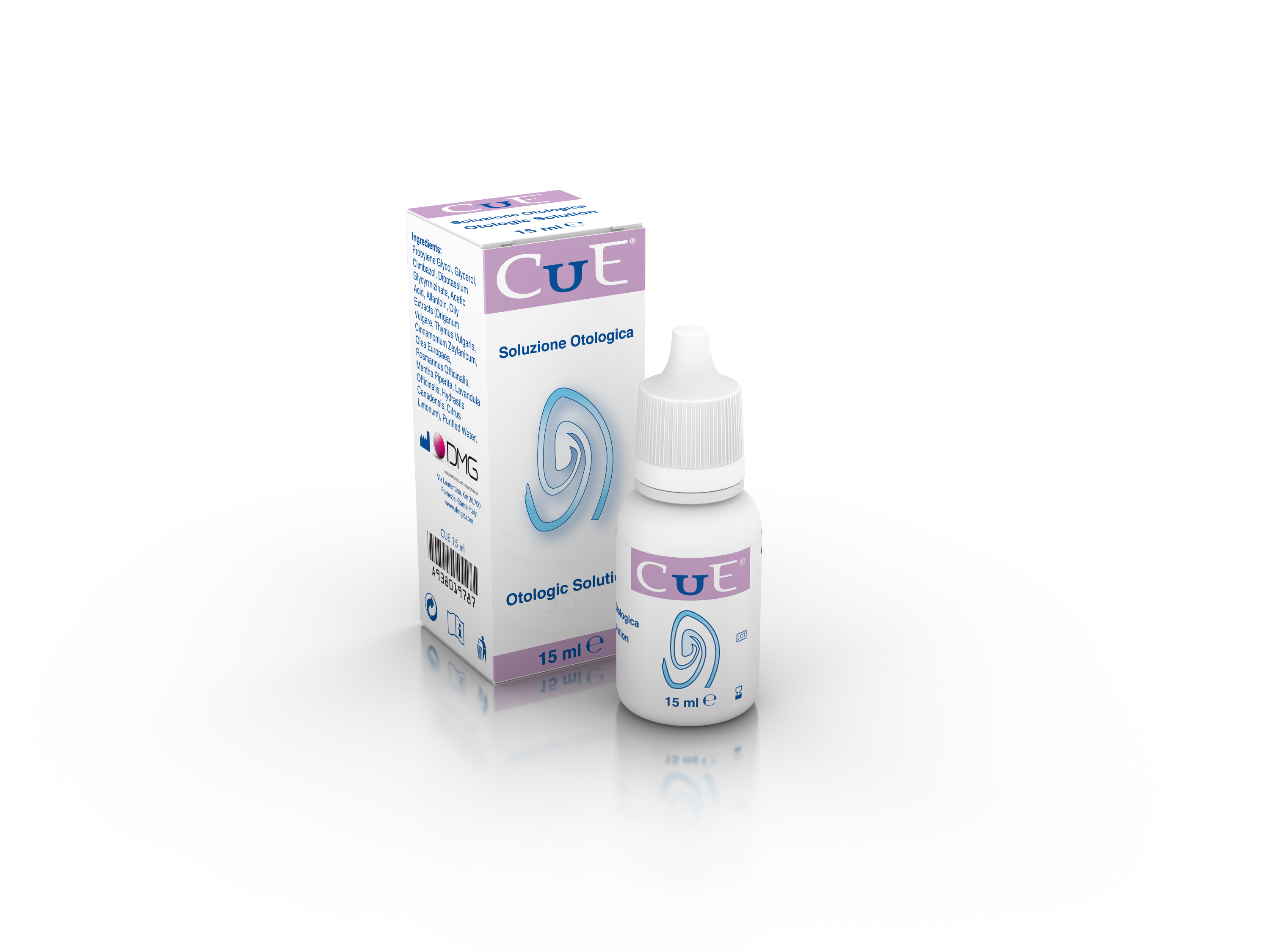
Product CUE - For the treatment of the irritations of the outer ear canal - Otologic Solution
Medical device for the treatment of the outer ear canal irritations.
Indications.
Cue Otologic Solution is a medical device with mechanical action. With its lubricant, emollient, protective and soothing properties, is indicatedboth in adults and children for preventing and helping the treatment o... -
Product CUE CAP - For the therapeutic management and the prevention of cerum plug - Ear Drops
Helps removal of cerumen from the external auditory canal. Before ENT visits or for hearing aid wearers.
Indications.
Cue CAP is an otologic solution to soften and facilitate the removal of the cerumen from the external auditorycanal. It is also indicated before ENT visits or prior to local d... -
Product COLINOX - Treatment of meteorism, aerophagy and flatulent colics in infants, children and adults - Gastric functional suspension
Colinox, an oral gastric-functional suspension, is a caramel-coloured emulsion containing Simethicone andBacillus coagulans, formulated in drops for easy administration.It is a mechanically-acting medical device. It acts by reducing the surface tension of gas bubbles that forminside the digestive apparatus... -
Product COLINOX - Treatment of meteorism, aerophagy and flatulent colics in adults - Chewable Tablets
Indications.
Indicated for the treatment of flatulence, aerophagy and gas colics in infants, children and adults, even in thepresence of changes in the intestinal bacterial flora.Indicated for dyspeptic disorders, in all cases requiring reduced gastric emptying times and in the treatmentof irritable b...
D.M.G. ITALIA S.R.L. resources (1)
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance