- Home
- Registrar Corp
- U.S. FDA Registration & Listings
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
U.S. FDA Registration & Listings

Product Description
Owners or operators of drug establishments that engage in the manufacture, preparation, propagation, compounding, or processing of drugs distributed in the United States are required to register and submit a list of every drug in commercial distribution in the United States. Establishments located outside of the United States must designate a U.S. Agent for FDA Communications. Registrar Corp's Regulatory Specialists can register your establishment with the U.S. FDA, act as your U.S. Agent, and list your devices. Our FDA Registration service comes with year-round benefits, and our 20 offices around the world will ensure you have access to localized assistance. Contact us to learn more. (Also available for Medical Devices)
Registrar Corp

-
US
-
2020On CPHI since
Categories
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Consulting Services
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Regulatory Affairs
- Packaging Materials and Equipment (Products) Printing / Labelling / Leaflets, Safety, Secondary Packaging
Sales Markets
- Middle East Region (e.g. UAE)
- Oceania
- North America (USA, Canada)
- Africa
- Central America (e.g. Mexico)
- East Asia (e.g. China, Japan, Korea)
- Europe - EU countries
- Europe - non EU (e.g. UK, Russia, ex-CIS countries)
- South America (e.g. Brazil, Colombia)
- South Asia (e.g. India, Pakistan, Sri Lanka)
- South East Asia (e.g. Thailand, Philippines, Singapore)
Registrar Corp

-
US
-
2020On CPHI since
More Products from Registrar Corp (2)
-
Product U.S. FDA Drug Labeling Assistance
The U.S. FDA requires that drug labels be indexed using Extensible Markup Language (XML) in Structured Product Labeling (SPL) format. Registrar Corp can help modify your drug labeling to comply with FDA regulations. Registrar Corp provides revised graphic files ready to be printed or edited, and a report t... -
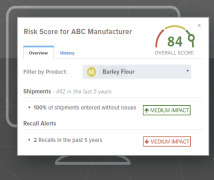
Product FDA Compliance Monitor (Quality Assurance Tool)
Automate your supply chain management. The FDA Compliance Monitor allows you to easily identify vulnerabilities in your supply chain. You can monitor the status of your own or your suppliers' FDA registration numbers as well as monitor for recalls, inspection results, warning letters, imp...
Recommended Products
-
Product IT/EU REGULATORY AFFAIRS - Support and management of Technical documenta...
OP Pharma has been providing innovative services in the Regulatory Affairs field for more than 20 years to small to big pharma Companies in Italy as well as in the European Union.
Our expertise covers the lifecycle management of pharmaceutical products, whole or in part, ranging from digitization ...
-
Product Regulatory & Market Access
With the growth in pharma manufacturing capability in Asia, each manufacturer nurtures an ambition to have their own footprint in the EU market.
PharmSol, with its own setups in Germany and Malta, has created a platform which provides a comprehensive, integrated and seamless market access and su...
-
Product Regulatory Affairs
The field of RA encompasses all the work necessary to manage products' registrations and to receive and maintain marketing authorization.Choose PQE Group's comprehensive support and broad strategic knowledge to launch products without delays and keep them on different worldwide markets.
-
Product Consulting: ICH Q10
Pharmaceutical companies must be capable of delivering products to the market with the utmost level of quality and safety. At Zamann Pharma Support, we have been aiding large and mid‐sized pharmaceutical companies in solving quality and compliance challenges while ensuring that practicality and qualit...
-
Product Development and engineering services by GEMÜ
GEMÜ engages with its customers at an early stage and supports them in all aspects of product development. From conceptual design, mould engineering, material consulting to sterilization and regulatory support, we can provide all competencies in-house. In addition, our developments are supported by sim...
-
Product Regulatory Sciences
From early-stage development to post-approval, we partner with pharmaceutical, biotechnology, and medical device clients to overcome regulatory hurdles. Using science as the driver for success, we help our clients achieve positive regulatory outcomes with the Food and Drug Administration (FDA), Europea...
-
Product Custom Protein Synthesis
We provide a Custom Protein Synthesis Service, using a chemical method that synthesises proteins amino acid by amino acid and making modifications on an atomic-scale. We work closely with our partners in designing, customising and optimising the proteins that is synthesized in an automa...
-
Product Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
AI-enabled, cloud-ready,360 pharmacovigilance solution for safety database management. An end-to-end globally compliant safety software for Drugs, Devices, Vaccines and Combinational products from case intake to submissions and reporting. • Case Intake • Case Processing • Case Submission • Risk Manage...
-
Product Toxicology
Our experts ensure clients' safety by protecting them from pharmaceutical hazards
At PharmEng Technology, we understand the importance of evaluating the toxicity and safety of biopharmaceuticals, therapeutic proteins, chemicals, active pharmaceutical ingredients, and components.
P...
-
Product QUALIPHARMA
Qualipharma is a consulting company created in 2001 and specialized in GMP&GDP advisory and consultancy services for healthcare and pharmaceutical sectors. Nowadays, our company is made up by a team of 80 experienced professionals that guarantees the highest quality customer service experien...
-
Product Auditing
Our pharmaceutical auditing and management services give you a transparent view of your supply chain enabling you to identify and mitigate the intrinsic risk in your operations, supply chains and business processes. Through our shared audit programs, delivered by our global network of specialist audit...
-
Product Value-added generic development
Avivia offers full product development services to generic drug companies and specialty pharma focused on the development of new improved versions (value-added generics or generic plus) of established drug products.
Although in general the overall aim of value-added generic development projects is focu...
-
Product Wasdell QP Services
Our dedicated QP Services team are able to provide compliance support, audit services, regulatory consultancy and QP certification for imported medicinal products.
-
Product Pharmaceutical drug substances (APIs)
Seratec specializes in fine organic chemistry and is dedicated to active pharmaceutical ingredient (API) development & production for the pharmaceutical industry.
Seratec operates in highly regulated markets and where high quality products are critical.
Seratec offers a complete range of cust...
-
Product Intra mammary and Oral Paste Veterinary syringe
Union Plastic offers a wide range of veterinary plastic products which includes intra mammary and Oral Paste syringe. Contact us for more information.
-
Product Analytical Development
Recipharm offer analytical support for drug discovery, pharmaceutical development and manufacturing through our global development facilities.
-
Product Regulatory Services
Our regulatory services team has decades of experience in obtaining and maintaining market authorizations and registrations. We ensure compliance of medicinal products, medical devices and food supplements during their complete life cycle. Currently we obtain compliance in 55 countries worldwide. Conta...
-
Product Research & Development of medical devices
We provide a complete and comprehensive range of services from A to Z for substance-based medical devices: from product idea, through development and research, certification, registration and product delivery to the customer.
With us, you can easily launch a new substance-based ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Pediatric Development
Pediatric formulation and product development
The development of acceptable, palatable pediatric formulations is a key feature within the industry today, driven by patient needs and regulatory requirements. Quotient Sciences has extensive knowledge and capabilities that enable us to provide you...
-
Product Biocatalyst production
Is the enzyme you are interested in not commercially available and a production needs to be developed? Enzymicals has extensive expertise with scale-up from lab to pilot-scale and technology transfers to industrial manufacturing sites. We can supply your protein by in-house production up to the kg-scale...
-
Product International Debt Collection
Debt collection activities all over the world: we support Top European Pharma Groups, with extra-performances -
Product AI and non-AI validation technologies for Life Science companies
We specialize in IA and non-AI Computer System Validation, equipment validation, utilities qualification, and IT/OT infrastructure qualification. Our services comply with the regulatory standards of the EMA, FDA, WHO, and other key Life Science authorities across Europe and the Americas. With a proven trac...
-
Product Regulatory Affairs & Compliance Support
Thépenier Pharma & Cosmetics offers comprehensive regulatory affairs and compliance services to ensure that all products meet global pharmaceutical standards. From dossier preparation to liaising with health authorities, the team ensures smooth regulatory approval processes. With expertise in GMP, ISO ...
-
Product ANDA and NDA Technical Support
As part of its commitment to full service support, Avéma Contract Services is pleased to offer our customers support with ANDA and NDA applications, including bio equivalency studies, collecting background data, supervising technology tranfers and reviewing applications to make sure that companies com...
-
Product Regulatory Affairs Services
Midas Pharma offers wide range of pharmaceutical services which includes regulatory affairs services. It offers regulatory support for human medicinal products, veterinary medicinal products and herbal medicinal products in Europe. This includes: • drug substance services: development of ASMF/E...
-
Product CordenPharma Drug Product Support Services
SUPPORT SERVICES FOR OUR CONTRACTED CUSTOMERS • Analytical Development • Validation Support • Regulatory Support • Clinical Supply Services
-
Product PeP11 LabCal Excel Platform
PeP11 LabCal offers a Single Validation SaaS Solution Platform that manages all Excel spreadsheets, eliminating the need for individual validations. This comprehensive electronic Excel solution features an Electronic Signature, Audit Trail, and Role-Based Access, rendering paper signatures unnecessary ...
-
Product Regulatory services
For pharmaceutical products, we offer support for regulatory services such as:
registration via national and European Union procedures;ANDA registration;
drafting of e-CTDs to create e-CTD sequences;
regulatory compliance;
minor and major variations (evaluation and/or management).For ... -
Product Cortellis HTA Intelligence™
Increase successful submission outcomes in markets worldwide with comprehensive, expertly analyzed regulatory and market access information:
-Get treatments to patients faster Compare and monitor regulations quickly across markets to understand regional health technology assessment (H...
-
Product Commercialization Services
CARBOGEN AMCIS has a track record, extending over 25 years, helping our customers transition their molecules from process development through validation and ultimately into commercial status. A range of services backed up with fully integrated support functions has been developed to facilitate and smooth t...
-
Product Labels for clinical studies
We offer a wide range of products which includes labels for clinical studies, special solutions for the clinical testing of pharmaceuticals, special multipage labels carrying test information, printing of fixed and variable data on the cover, removable labels can be positioned on medical records. Contact u...
-
Product Web 4.0 5D
The latest version of Web 4.0 CMYK full color printer represents the state of the art for inline digital printing and inspection of continuous web packaging materials. Printing in vivid color and precise details on a wide range of substrates with high reliability and maximum process control, the Web 4.0 5D...
-
Product Smart and Intelligent Packaging
Smart and intelligent pharmaceutical packaging refers to the integration of advanced packaging technologies to enhance the safety, efficacy, and convenience of pharmaceutical products. A variety of technologies can be used to create such packaging, including RFID, NFC, temperature indicating inks, battery-...
-
Product Leaflets
FEATURES- Single and multi-color options
- Good opacity (low degree of transparency)
- Sufficient degree of whiteness
- Good printability
- Codability
- Secure monitoring in the folding process to prevent mix ups
- Highest requirements of pr...-comp313163.png)
-
Product Booklet & multi-page labels
Cinta-Plast,S.A offers a wide range of multi-page and booklet labels.Add extended content on unique label in a reduced area.
Customizable with Pharma material range.
Contact us for more information.
-
Product ETIQUETTE MEDICAMENT
Une étiquette experte qui répond à l'exigence pharmaceutique de :
. Précision,
. Dosage,
. Graduation,
. Sécurité,
. Résistance : passage en autoclave,
. Performance à la dépose automatique à grande vitesse.
. Garantie
. Respect de conformité au normes…
...
-
Product Cold Chain
Cold chain monitoring and compliance is essential for the effectiveness of many products.
Every product has different characteristics and for this reason we have studied customized solutions:
• Programmable temperature sensors. • Temperature-sensitive magnetic inks. qq...
-
Product SELF ADHESIVE HANGING DEVICE
The Self Adhesive Hanging Device for infusion bottles is one of the latests Pilot Italia patents.
Our R&D team has developed and patented three labels with an innovative handle and various functionalities, aimed at simplifying and supporting operating room staff while meeting custome...
-
Product Security Label (Tamper Evident)
Tamper-evident labels provide visual evidence that the container has been tampered with or opened. Unlike shrink sleeve types, our Shrink Tack Label does not require a heat tunnel, in which heat is applied to the whole container. Thus, there is minimum heat impact on your product. Tamper evident label ...
-
Product PHL Isolator - Pharma Hospital Laboratory Isolator
Thanks to its innovative characteristics and flexibility, the new Pharma Hospital Laboratory Isolator (PHL) is suitable for different applications, such as preparation of cytotoxic products, manipulation of hazardous ingredients and cellular labelling. The operator may operate completely isolated f...
-
Product Consultancy
MFT Consult, our team combines deep expertise in regulatory compliance, facility design, QMS, and technology transfer with the ability to act quickly and efficiently. With decades of experience in the pharmaceutical and biopharmaceutical industries. we specialize in guiding startups in the pharmaceutical a...
-
Product Leaflets
The packaging insert is an important part of the secondary packaging, following the need that every medicine has to be delivered with comprehensive information about intake, side effects and more towards the customer. In every target market there are specific regulatory requirements to follow leading to a ...
-
Product Labelling for Clinical Trials - Booklets | Multi-Layer | Tamper Evident ...
INOVALABEL PHARMA COMPETENCE
Inovalabel specialises in complex multi-page labels and customised special designs.
Years of experience and intensive customer exchange have made Inovalabel one of the most competent experts in the field of labelling for Clinical Trials.
...
-
Product Unique medical leaflet - Thin Bible Paper Booklet
In pharmaceutical industry, product leaflets in a package are over 100y old innovation with several practical issues (usability, number of languages, sustainability etc.). We have outstanding unique new solution for this!
Unique medical leaflet - Thin Bible Paper Booklet is the new standard...
-
Product H-Track Laser Writer Machine
This machine is designed to laser etch a trademark onto a single surface of a soft gelatin capsule. Servomotors and the cantilever system simplify precision laser writing registration. Integrated controls and quick change parts make this machine user-friendly for both operation and maintenance. Contact us ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































.jpg)