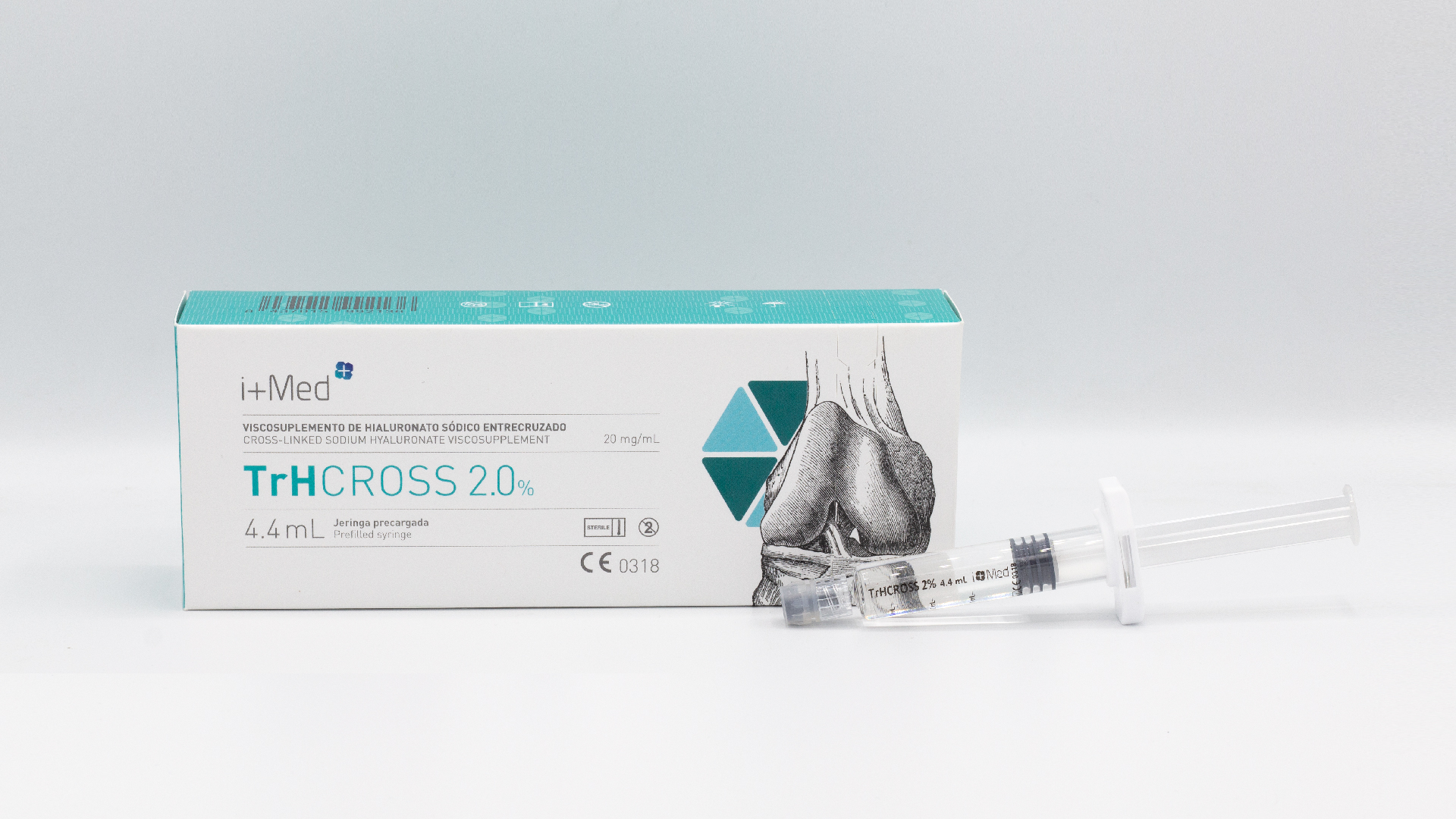
TrHCROSS - Joint injections (cross-linked HA 2%)
Product Description
i+Med S. Coop.

-
ES
-
2018On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
i+Med S. Coop.

-
ES
-
2018On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from i+Med S. Coop. (10)
-
Product BtHLINE and BtHCROSS - Dermal fillers for aestheic medicine (Linear HA 1,4%, Crosslinked HA 1,5%, 1,75%, 2%)
BtHLINE 1,4% is a biocompatible linear hyaluronic acid hydrogel intended as a dermatologicaltreatment for aesthetic purposes. Administered by intradermal injection.
Composition
Non-animal HA Low level of endotoxins
Properties
BtHLINE 1,4% + Restores aged skin structures. ... -
Product Daydrop - controlled release multidose eye drops with ectoine for dry eye
DayDrop is an eye drop indicated to relieve dryness and eye irritation caused by environmental factors (wind, dust, smoke, air conditioning, dry heat), prolonged use of screens or contact lenses and / or certain ocular pathologies that occur with these symptoms.
Hydrates, lubricates and protects for... -
Product DayDrop - eye drops with 12h sustained-release system of ectoine
DAYDROP. INNOVATIVE FORMULA WITH HIALURONIC ACID AND 12h SUSTAINED RELEASE OF ECTOINE Eye drops indicated to relieve dryness and eye irritation caused by environmental factors (wind, dust, smoke, air conditioning, dry heat), prolonged use of screens or contact lenses and / or certain ocular pathologies th... -
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.).... -
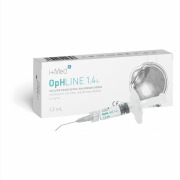
Product OpHLINE - Ophthalmic Viscosurgical Devices (HA 1,4%/2%/3%)
OpHLINE is an ophthalmic viscosurgical devise (OVD) indicated as a coadjuvant during surgical procedures, involving the anterior chamber of the eye, especially in cataract surgery, including the extraction of the lens and the insertion of intraocular lenses.
OpHLINE is available in 3 models ... -
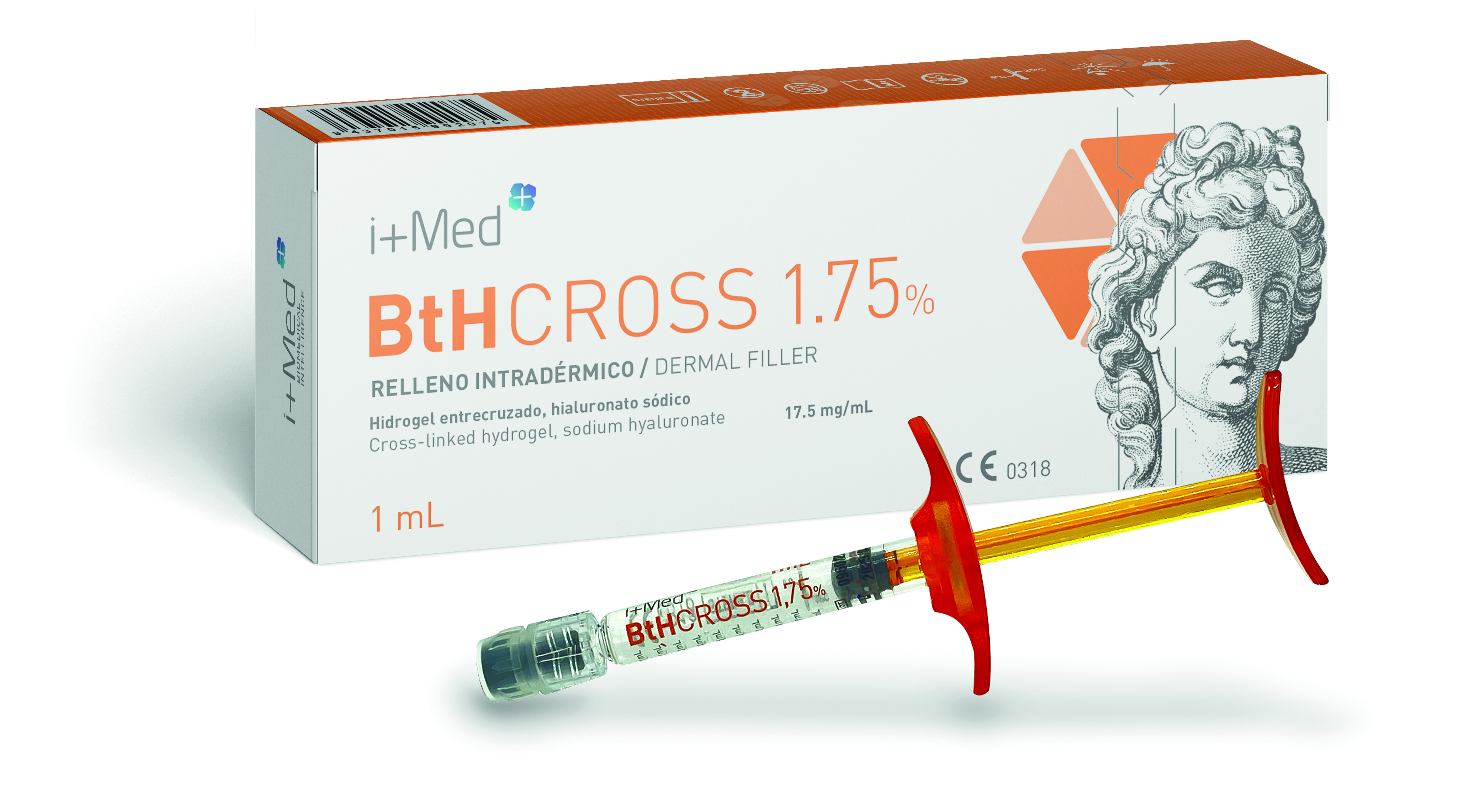
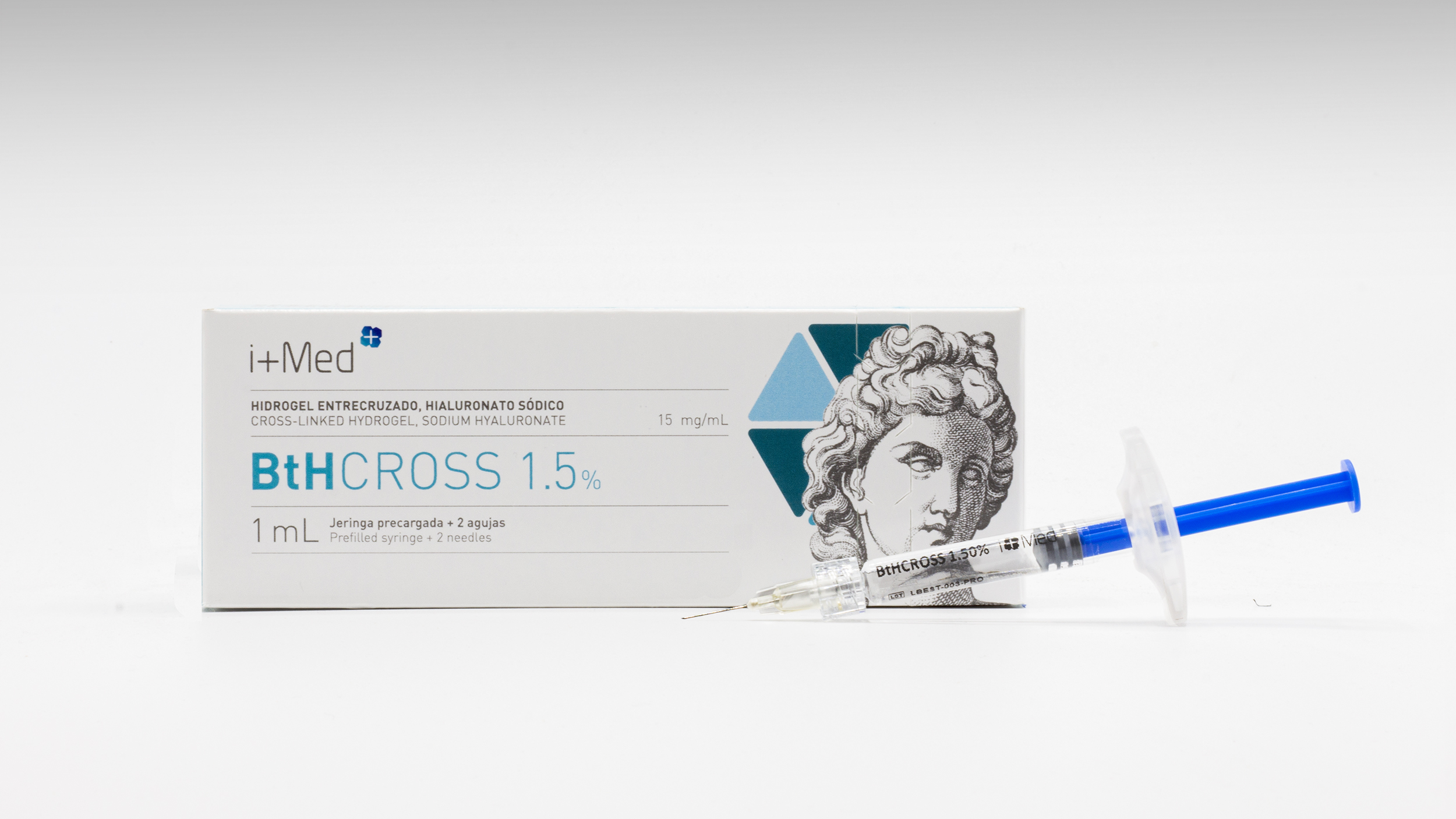
Product BtHCROSS - Dermal filler (cross-linked HA 1,5%/1,75%/2%)
BtHCROSS is a biocompatible cross-linked hyaluronic acid hydrogel intended as a dermatological treatment for aesthetic purposes. It is administered by intradermal injection and designed to restore and correct facial volumes and fill wrinkles and folds.
Depending on the depth of the wrinkles an... -
Product DayDrop - eye drops with the sustained-release system of ectoine
DayDrop is an ophthalmic solution with sustained release of ectoine, indicated to alleviate the ocular dryness and irritation produced by environmental factors (wind, dust, air conditioning, dry heat), prolonged use of screens or contact lenses and some pathologies that present the above mentioned symp... -
Product Profilm COLD SORES - protective film for cold sores(herpes)
Profilm COLD SORES is a liquid dressing for topical use, indicated to relieve the initial symptoms of cold sores. Its technology firms a protective and discreet physical barrier that adapts to the shape of the skin, reducing the risk of infection and the spread of the virus. Due to its composi... -
Product Profilm MOUTH ULCERS - protective film for mouth ulcers and aphthae
Profilm MOUTH ULCERS is a hydrogel, supplemented with natural extracts of propolis and tea tree, and enriched with stabilized vitamin C. After its application, and thanks to its technology, a protective physical barrier is formed which adapts to the shape of the mucosa, preventing contact with... -
Product BuddyDrops - eye drops with ectoine and HA for veterinary use
BuddyDrops is a sterile moisturizing and lubricating long-lasting ophthalmic solution intended for veterinary use. BuddyDrops increases the contact time of the drop on the ocular surface reducing the need for repeated applications of the product and thus improving therapeutic compliance.
...
i+Med S. Coop. resources (14)
-
Brochure i+Med Company profile
i+Med is a biotech company formed by highly qualified personnel, focused on the R&D and manufacture of new products for third parties. Our ADAPTEK® TECHNOLOGY enables the development of customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.). These intelligent nanoparticles could be programmed to respond to external stimulus (E.g. pH or t˚) that allow the controlled release of APIs. As a result, we can develop high added value and differentiated products that provide our customers with a key competitive advantage in t...
-
Video Company video presentation
i+Med is a company involved in the research, development and manufacture of new products in the field of biomedical engineering.
We specialize in the generation of intelligent Nanohydrogels and customized smart systems for controlled release adapted to the needs of each client, always under a strict control of the manufacturing process and a demanding quality policy.
More information at https://imasmed.com/en/ -
Brochure OpHLINE - Ophthalmic Viscosurgical Devices (HA 1,4%/2%/3%)
OpHLINE is an ophthalmic viscosurgical devise (OVD) indicated as a coadjuvant during surgical procedures, involving the anterior chamber of the eye, especially in cataract surgery, including the extraction of the lens and the insertion of intraocular lenses.
OpHLINE is available in 3 models with different concentrations (1,4%, 2%, 3%) and properties to provide better options for a variety of surgical needs.
Properties
+ Lens removal.
+ IOL implantation.
+ High protection of the endothelium.
+ Total and safe extraction.
+ Excellent visibility of the operating field.
+ Sterilization by moist heat.
Packaging
OpHLINE is supplied as a Pre-Filled Syringe on apyrogenic Type I glass, ready to use. A single use injection cannula is supplied as accessory.
-
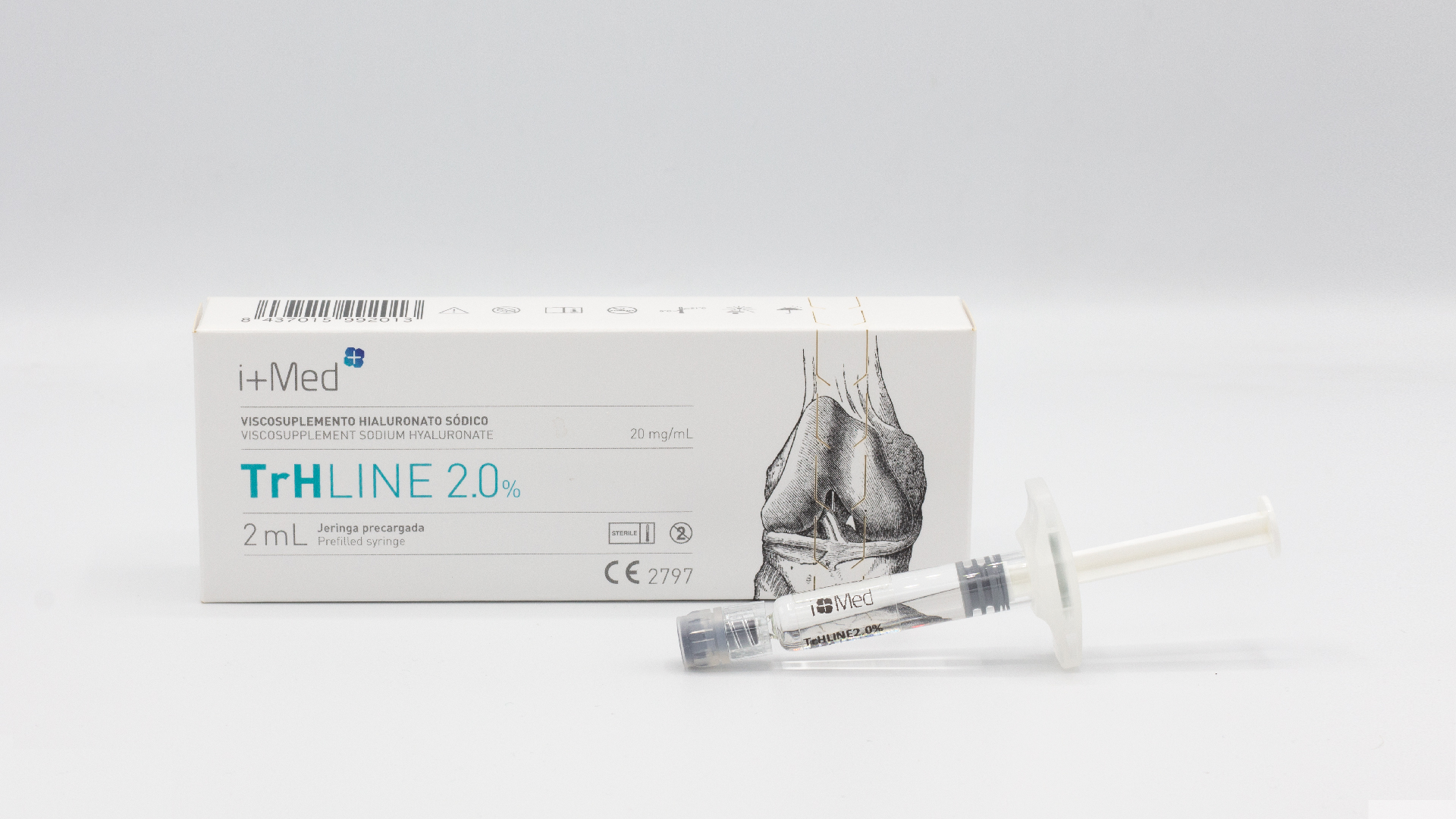
Brochure TrHLINE - Joint injections (linear HA 1%/2%)
TrHLINE is a linear hyaluronic acid injection, designed for intra-articular administration to restore the viscoelastic properties of synovial fluid in traumatic and degenerative lesions of the joints, manifested by pain syndrome and reduced mobility.
TrHLINE is available in 2 models with concentrations of 1% and 2% to select the most appropriate treatment for each patient.
Properties
+ Lubricating effect.
+ Shock absorption.
+ Reduction of pain in the joint.
+ Improves mobility and quality of life.
+ Increases the functionality of the joint.
+ Easily reabsorbed by the body.
Packaging
TrHLINE is supplied in a pre-filled glass syringe of 2 mL, ready to use.
-
Brochure TrHCROSS - Joint injections (cross-linked HA 2%)
TrHCROSS 2% is a biocompatible hydrogel of cross-linked sodium hyaluronate obtained by biofermentation. This is a single injection, designed to relieve joint pain in osteoarthritis by replacing natural joint fluid and replenishing lubricating and shock-absorbing functions.
Properties+ Reduces the pain and increases the functionality of the joint.
+ Lubricating and shock-absorption effect.
+ Improves mobility and quality of life of the patient.
PackagingTrHCROSS is supplied in a pre-filled Type I syringe of 4,4 mL, ready to use. It is presented in an individual blister along with four control labels and instructions for use. -
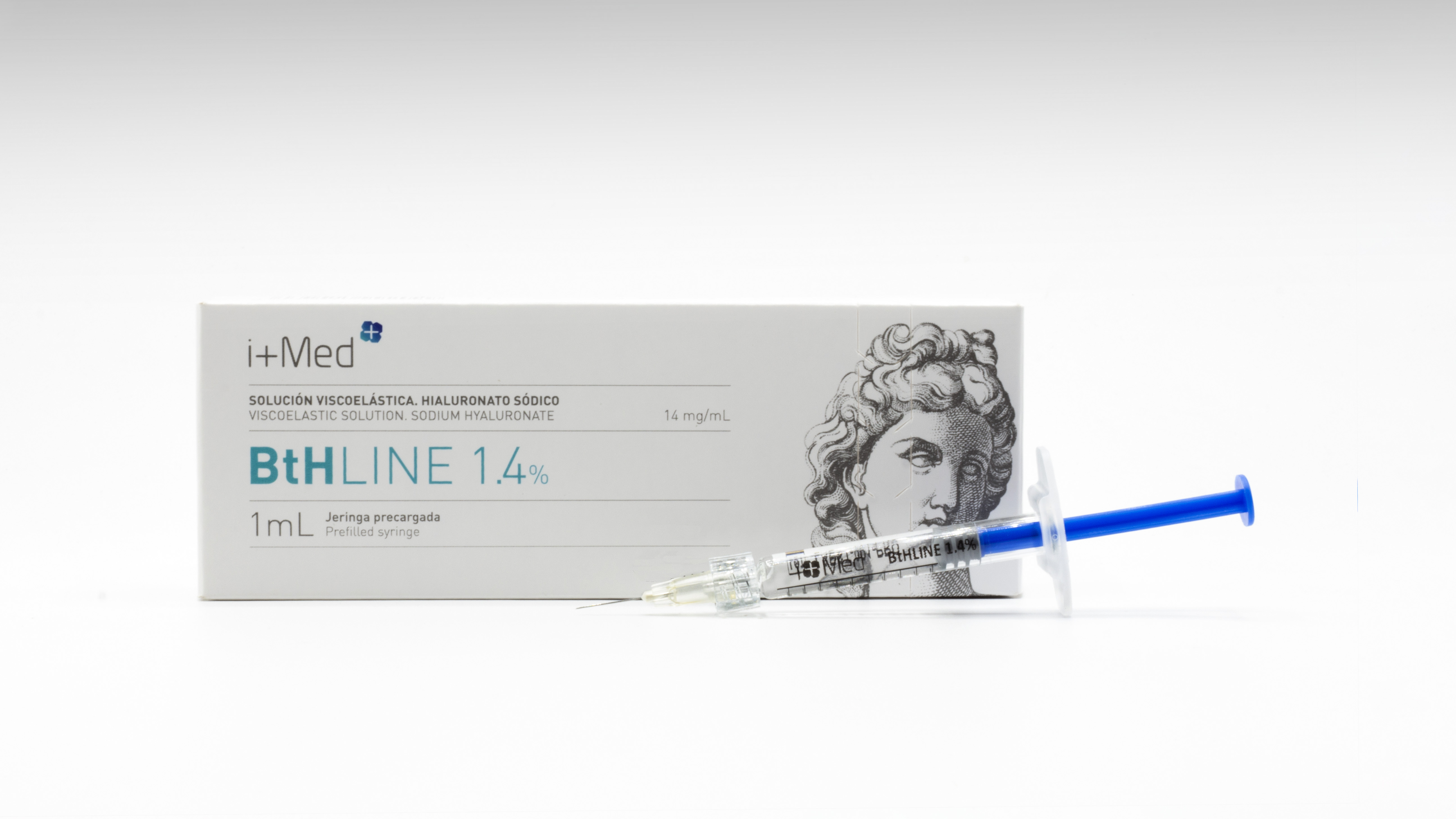
Brochure BtHLINE - Dermal filler for biorevitalization (linear HA 1,4%)
BtHLINE is a biocompatible linear hyaluronic acid hydrogel intended as a dermatological treatment for aesthetic purposes. Administered by intradermal injection, the product is indicated to complement the extracellular matrix and intradermal tissue, restoring the anatomical structures of the skin, promoting its hydration, revitalization and toning.
Properties
+ Restores aged skin structures.
+ Improves skin hydration.
+ Revitalizes skin and improves its firmness.
Packaging
BtHLINE is presented in a pre-filled syringe of 1mL, ready for use. It is supplied in a single blister together with two sterile needles (30G) for single use, as accessories.
-
Brochure BtHCROSS - Dermal filler (cross-linked HA 1,5%/1,75%/2%)
BtHCROSS is a biocompatible cross-linked hyaluronic acid hydrogel intended as a dermatological treatment for aesthetic purposes. It is administered by intradermal injection and designed to restore and correct facial volumes and fill wrinkles and folds. -
Brochure DayDrop - eye drops with the sustained-release system of ectoine
DayDrop is an ophthalmic solution indicated to alleviate the ocular dryness and irritation, produced by environmental factors (wind, dust, air conditioning, dry heat), prolonged use of screens or contact lenses and some pathologies that present the above mentioned symptoms. -
Brochure Profilm MOUTH ULCERS - protective film for mouth ulcers and aphthae
Profilm MOUTH ULCERS is a hydrogel with a polymeric base, supplemented with natural extracts of propolis and tea tree, and enriched with stabilized vitamin C. After its application, a protective physical barrier is formed which adapts to the shape of the mucosa, preventing contact with food, saliva and other irritant or sensitizing elements or substances. Provides anti-inflammatory and analgesic effects, creates an environment that favours the healing of injuries.
Properties+ Forms a protective film that remains on the site of application, promoting healing.
+ Relieves pain and protects / isolates against external agents.
+ Reduces the extension and duration of canker sores.
+ Contains propolis and tea tree extracts, stabilized vitamin C.
Packaging
Profilm Aftas Bucales is presented in a 5 mL airless container. -
Brochure Profilm COLD SORES - protective film for cold sores(herpes)
Profilm COLD SORES is a liquid dressing for topical use, indicated to relieve the initial symptoms of cold sores. Its technology firms a protective and discreet physical barrier that adapts to the shape of the skin, reducing the risk of infection and the spread of the virus. Due to its composition, Profilm conditions the skin and helps to relieve the initial symptoms of the herpes outbreak (such as tingling, pain, itching or burning in the area).
Properties+ Forms an invisible film that adapts to the shape of the skin.
+ Relieves the first symptoms of cold sores and reduces the duration of the outbreak.
+ Reduces the risk of infection and contagion of the virus.+ Relieves itching and pain.
+ Contains propolis and tea tree extracts, stabilized vitamin C
Packaging
Profilm Herpes Labial is presented in a 5 mL airless container. -
Brochure BuddyDrops - eye drops with ectoine and HA for veterinary use
BuddyDrops is a sterile moisturizing and lubricating long-lasting ophthalmic solution intended for veterinary use. BuddyDrops increases the contact time of the drop on the ocular surface reducing the need for repeated applications of the product and thus improving therapeutic compliance.
Properties
+ Alleviates dry eye and dry eye sensation.
+ Alleviates eye irritation.+ Protects against external allergens alleviating allergy symptoms.
+ Regenerates and stabilizes the tear film.
Packaging
BuddyDrops is presented in a sterile 15 ml plastic dropper vial.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













2-file108437.jpg)
-file108440.jpg)




