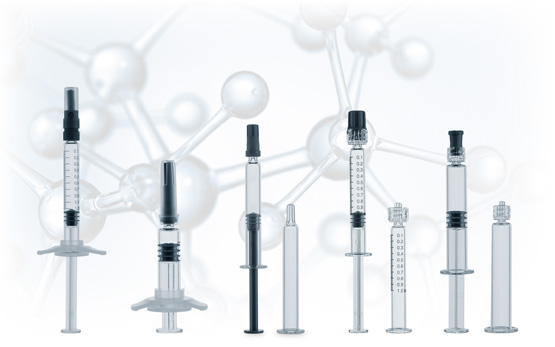
Syringes for sophisticated drug products
Product Description
Gerresheimer AG

-
DE
-
2015On CPHI since
-
2Certificates
-
5000+Employees
Company types
Primary activities
Categories
Specifications
Gerresheimer AG

-
DE
-
2015On CPHI since
-
2Certificates
-
5000+Employees
Company types
Primary activities
More Products from Gerresheimer AG (25)
-
Product Primary Packaging Glass - RTF vials
Gx® RTF vials enhance flexibility by facilitating packaging needs from clinical stage to industrialization with the common goal to minimize customer product risks and optimize total cost of ownership. Gerresheimer combines the competencies in converting glass tubes to serum/ injection vials and tra... -
Product Primary Packaging Glass - Gx® Elite Glass Vials
The Gx Elite vials are the result of a careful product development process spanning several years. The highly shatter-resistant vials are extremely durable and free of cosmetic defects. They also boast an incredibly robust structure, while their resistance to delamination protects the drug inside. Simp... -
Product Primary Packaging Glass - Vials
Our tubular vials product range includes clear and amber vials in glass types I, II and III from 0.6 to 100 ml. We produce different designs, with or without blowback, according to industry standards or to individual specifications. As a special feature we offer sterile RTF® vials based on its long e... -
Product Primary Packaging Glass - Cartridges
Gerresheimer produces high-quality cartridges with fill volumes ranging from 1.5 to 3.0 ml (sizes up to 10.0 ml on request) for various pharmaceutical applications. Our product range covers clear and amber cartridges made of borosilicate glass of hydrolytic class I. Individual options, such as an a... -
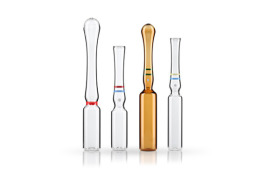
Product Primary Packaging Glass - Ampoules
Gerresheimer offers a broad portfolio of highly competitive pharmaceutical ampoules in type I pharma glass. Our standard range covers ampoules made of flint and amber glass with filling capacities from 1 to 30 ml. These include straight-stem, funnel-type and closed ampoules of ISO types B, C and D ... -
Product Primary Packaging - Gx® prefillable syringes
Prefillable glass syringes in bulk and "ready-to-fill" format, type 1 glass, filling volumes of 0.5 ml to 5.0 ml, luer cone and luer lock syringes, needle syringes, silicone-oil reduces Gx Baked-on RTF® syringes, metal-free Gx RTF® syringes, innovative syringe components, round and cut finger flange, most ... -
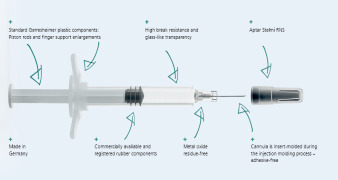
Product Gx RTF® metal-free glass syringes
One problem associated with syringe use is that traces of tungsten or other metals occasionally leave residue behind in the bore when the syringe cone is shaped. Gerresheimer developed a production technology registered for patent where the pin used to shape the cone is made of a special ceramic. So we can... -
Product Gx RTF® ClearJect® Polymer syringes
Gx RTF® ClearJect® syringes are made from COP (Cyclo Olefin Polymer). They are glue-free as the cannula is not glued in, but is instead insert-molded during the injection molding process, no aggregation due to metal or tungsten residues, high viscosity silicone oil for reduced particles, minimal extractabl... -
Product Drug Delivery Systems - Inhalers
In close cooperation with leading pharmaceuticals companies, Gerresheimer Medical Systems has been developing and producing powder inhalers, capsule inhalers and nebulizers for the treatment of respiratory illnesses like asthma, COPD (Chronic Obstructive Pulmonary Disease) and cystic fibrosis for m... -
Product Drug Delivery Devices - Pen injectors
In the pen injector product segment, our core competence lies both in high quality, reusable pen injectors and in economically produced disposable pens, including glass cartridges. Our many years of experience in the segment of „lancing devices and lancets" is especially advantageous here. We activel... -
Product Gx® AdhereSmart
Monitoring oral medication adherence in clinical trialsAdherence Tracking:
Smart cap tracks and sends patient’s adherence data
Real-time Monitoring:
Patient adherence is monitored, and deviations flagged
Corrective Measures:
Patients receive a reminder when they miss their medica... -
Product Velocity® Vials
Make the fill & finish process up to 50% more efficient with vials with a Velocity® coating. A major problem in the fill & finish process of conventional borosilicate glass vials is the friction caused by glass-to-glass and glass-to-metal contacts. Frictional resistance reduces the t...
Gerresheimer AG resources (40)
-
News Gerresheimer predicts weight-loss drug deals to account for 4% of yearly growth
Dietmar Siemssen, CEO of German primary packaging manufacturer Gerresheimer, states that approximately 4% of the company’s revenue growth each year to come from deals with drugmakers of weight loss and diabetes products, particularly GLP-1 class drugs. -
Brochure Primary Packaging Plastics - Solid dosages
Gerresheimer Primary Packaging Plastics specializesin the widely varied spectrum of pharmaceuticalplastic packaging within solid, liquid andophthalmic applications, plastic vials and plasticbottles for personal care. -
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delivery. -
Whitepaper Biological Solutions
Dedicated primary packaging for biological active ingredients : from reduced silicone- , and polymer syringes over ready-to-fill glass- and COP vials to customized product developments and much more.
-
News On track at Pharmapack 2024 - The Track Sponsor interview: Gerresheimer
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Gathering the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 offers exciting opportunities to learn and collaborate. -
Brochure Primary Packaging Plastics - Ophthalmic/nasal applications
Gerresheimer Primary Packaging Plastics specializesin the widely varied spectrum of pharmaceuticalplastic packaging within solid, liquid andophthalmic applications, plastic vials and plasticbottles for personal care. -
News COP Vials: Ice-cold solution for the challenges of biologics
Gerresheimer, innovative system and solution provider and global partner for the pharma and biotech industry launches with COP (Cyclic Olefin Polymer) vials a particularly satisfactory solution for the filling and storage of highly sensitive biologics. They are suitable for mRNA active ingredients. -
Brochure Customer Newsletter
Latest news and innovations in the world of Gerresheimer. -
News Gerresheimer Gx Elite Vials: safely putting production in the fast lane
Gerresheimer, an innovative system and solution provider and a global partner for the pharma and biotech industry, is presenting Gx Elite, a product line of glass injection vials designed to maximize patient safety and production efficiency. Superior quality and shatter resistance are the key priorities of the range. The advanced primary packaging solution is particularly suitable for sophisticated drugs such as biopharmaceuticals and cytostatics. Gx Elite vials can also be delivered pre-sterilized, ready-to-fill in Gerresheimer’s innovative and sustainable EZ-fill Smart packaging, for example. Gerresheimer will be presenting these and other solutions at CPHI Barcelona from October 24 - 26, 2023, at booth 50 in hall 2. -
Brochure Primary Packaging Plastics - PET for liquid and solid
Gerresheimer Primary Packaging Plastics specializesin the widely varied spectrum of pharmaceuticalplastic packaging within solid, liquid andophthalmic applications, plastic vials and plasticbottles for personal care. -
Brochure Primary Packaging Glass - Moulded Glass
Moulded Glass Packaging Solutions -
Brochure Primary Packaging Glass - Gx® Ready-to-fill Vials
Gx® RTF vials are washed, packed in trays or in a nest and tub, sterilized and delivered to pharmaceuticals customers. They can then immediately begin filling without further procees steps. Gx RTF® Vials: flexibility through different packaging configurationshighest quality requirementshighest standards with regard to glass breakage, cosmetic defects, paricle contaminationDelivered sterilePackaging based on Ompi EZ-fill packaging formats: identically packaged sterile vials from to different manufacturers -
Brochure Primary Packaging Glass - Glass Cartridges for Parenteral Drugs
Gerresheimer is regarded as a technology leader and ranks among the leadingcompanies in the world market. We offer a comprehensive range of cartridgesfor various pharmaceutical applications. Individual treatments complete the range.These include, for example, ammonium sulfate treatment to improve theglass quality in selected sites. -
Brochure Primary Packaging Glass - High-Quality Gx Glass Vials
Gerresheimer is regarded as a technology leader andranks among the leading companies in the world market.We offer a comprehensive range of vials from 0.6 to 50 ml in different designs, with or without blowback (European and American version) according to industry standards or to your individual specifications. State-of-the-art production process and in-line camera inspection systems ensure high-performance in filling lines and lyophilization processes. -
Brochure Primary Packaging Glass - Metal-free glass syringes
One problem associated with syringe use is that traces of tungsten or other metals occasionally leave residue behind in the bore when the syringe cone is shaped. Gerresheimer developed a production technology registered for patent where the pin used to shape the cone is made of a special ceramic. So we can offer a metal-free 1 ml Gx RTF® Luer lock glass syringe. The process can be transferred to other Luer lock syringe sizes or to Luer cone syringes of various sizes at any time. -
Brochure Primary Packaging - RTF® ClearJect® polymer syringes
The syringes are made from COP (Cyclo Olefin Polymer). The needle syringes are glue-free as the cannula is not glued in, but is instead insert-molded during the injection molding process. No aggregation due to metal or tungsten residues, high viscosity silicone oil for reduced particles, minimal extractables and leachables, high pH tolerance, no shift in pH value, optimized permeation rate, high break resistance, tighter tolerances than glass, precise medication dosing thanks to minimal residue volume, wide variety of design options and high flexibility for customer-specific requirements, outstanding syringe functionality with regard to break loose, gliding forces and pull-off forces of the needle shield, compatible with auto injectors. -
Brochure Primary Packaging Glass and Plastic - Gx® prefillable syringes
Prefillable glass syringes in bulk and "ready-to-fill" format, filling volumes of 0.5 to 5.0 ml, luer cone and luer lock systems, needle syringes, silicone-oil reduced and metal-free syringes for sensitive active ingredients, innovative syringe components. -
Brochure Primary Packaging Glass - Gx InnoSafe® syringes
With Gx InnoSafe, Gerresheimer offers a syringe with an integrated safety systems that prevents unintentional needle stick injuriesexcludes the possibility of accidental reuse andtakes the needs of pharmaceutical companies and end users equally into account. -
Brochure Customized Drug Delivery Systems, Medical and Diagnostic Products
From the concept development through industrialization to assembly, and pharmaceutical filling. We cover the entire spectrum of delivery types: inhaler, syringes, pen systems, auto injectors and infusion sets
-
Video Exploring Platform Solutions: From Primary Packaging to Advanced On-Body Drug Delivery Systems
Holger Krenz will be speaking at CPHI Milan about Gerresheimer’s platform solutions for primary packaging and drug delivery systems. His presentation will focus on the product benefits of the Gx Elite performance level which are the ideal packaging components for reliable functionality of drug delivery systems. -
Video Gx Elite RTF® Glass and Polymer Syringes – First Choice for Demanding Biologics
Ensure highest product quality and lowest cosmetic defects Accelerate drug development by most comprehensive data packages Create tailored system options with state-of-the-art technologies and components Offer best fit for demanding drug formulations by addressing bio-pharmaceutical requirements -
Webinar Gerresheimer's Product Platforms: Designated Quality Levels to Secure Best in Class Performance in Multiple Application Fields & Demanding Disease Areas
"As a solution provider with a unique & dedicated product portfolio, Gerresheimer is continuously exploring options to better accommodate their customers along the drug development and drug LCM journey. With its newly installed product platforms, Gerresheimer will be combining outstanding quality levels with specific product requirements in dedicated application fields and disease areas. From dental care over hematology, vaccines, diabetes and metabolism disorders into complex and more demanding areas such as Cell Gene Therapy, ophthalmology and oncology ... Gerresheimer will be able to support even better in finding the best containment solution for their specific application. Picking one example, we'll elaborate on our offering in the ophthalmology field ..." -
Webinar Keynote Address
How can device and packaging innovation influence patient ability and motivation to use their treatment?" With examples from research of how patients learn and become motivated, and how industry can use these processes to influence patient outcomes. Patients learn through a mixture of stimuli and learning processes, these stimuli can be adopted into device design and packaging to enhance the patient's feelings about their medication. Presently many patients using complex devices, like inhalers, make critical errors which entails repeated re-training. This presentation looks at innovative ways of embedding learning, that could be adopted by future device and packaging design. -
Webinar Lightning Talk - Blister Packs - How is the Past shaping the Future?
What are blister packs, and why are they so important? How have they changed over the years, and where are they heading? This presentation will consider a history of how blister packs have benefitted the supply of medicines to patients worldwide, and how they are continuing to evolve within an increasingly sustainable landscape. -
Webinar Lightning Talk - Next-Gen Wearable Devices
Wearables can take different shapes and sizes and are revolutionizing the way we monitor, treat, and manage health conditions. In this short deep dive, we will look at innovative wearable technologies and how they enable data collection and analysis, patient centric care, personalized medicine and more. -
Webinar Partner Presentation - Gerresheimer
Overcoming challenges when developing subcutaneous drug delivery devices that empower patient self-administration. The trend in parenteral patient therapy has been moving towards the home-care setting for some time as it empowers patient independence. However, home administration brings additional requirements when developing a delivery device, and these are constantly evolving. Patient safety is always paramount, but usability is another critical factor, as well as sustainability and connectivity to support patient adherence. The challenge is to design a device to meet as many requirements as possible that can be ready on the market within 4-8 years. In this presentation we will discuss Gerresheimer’s mission of “innovating for a better life” and how we apply this when developing solutions that respond to the needs of patients, our pharmaceutical industry customers, and the environment, today and into the future. -
Webinar Lightning Talk - Opportunities and Challenges in Connected Drug Delivery
The talk explores the current integration of digital health in drug delivery and various opportunities that it presents. Delving into the landscape of drug delivery, we examine the rise of connected devices, emphasizing the delicate balance between technology and compassionate care. This session also addresses real-world challenges like adoption, satisfaction, regulatory, and security considerations. Be part of the conversation on overcoming these challenges. -
Webinar Sensory Exploration: A Journey into Patient-Centric Perception
From sight, touch, taste, and smell to hearing, the five senses allow the body to take everything in. Research shows that the more senses are used, the better the memory is gathered, and the process enhanced. How does this theory apply to device development, design conception, and patient experience? -
Webinar Connecting the Dots: Creating a Patient-Centric Supply Chain
The changing healthcare system increases the necessity for a more patient-centric approach. Join patient advocate Heidi Floyd, where she will delve into how pharma can create a robust patient-centric supply chain, such as:
Developing greater collaboration and communication between suppliers, manufacturers, and distributors to help manage stock constraints that affect end-users Leveraging the latest technologies to help suppliers source the right materials and manage demands Why mapping out key points in the supply chain lifecycle can help identify integration areas for patient involvement -
Webinar Creating Better Patient Experiences with Next-Gen Technology
In a world where patients are more than ever actively and digitally involved in their health, they demand experiences that are not inferior to the preferred experiences offered in other parts of their lives. This profoundly impacts packaging, clinical trial enrolment, and data capturing… Christophe highlights concepts worth considering when innovating your patient solutions in this talk. -
Webinar Panel: Improving the Patient Experience in Clinical Studies
Improving the patient experience in clinical studies is essential for encouraging participant engagement, retention, and satisfaction, ultimately leading to more reliable and meaningful research outcomes. Join our panel on for insights into improving patient outcomes in clinical studies View ‘The Patient Experience’ holistically during study design Incorporate the right technologies to minimize patient burden Solicit patient input for your study protocol Effectively bridging the gap between patient and manufacturer to improve the outcome of clinical studies -
Webinar Advancing Remote Patient Monitoring with Drug Delivery Devices and Digital
Remote Patient Monitoring has been growing steadily since COVID-19 as it allows healthcare providers to monitor patient health from the comfort of their home. Discover how a drug delivery device company can harness the power of digital technology in transforming patient care and creating opportunity for pharmaceuticals sector. Join us as we explore this topic, where we cover:
Making patient, physician and caregiver engagement a priority Unveiling RPM codes and discuss implementation challenges Explore the potential value generated of remote patient monitoring for patient, physician and pharma -
Webinar Developing Packaging with Your Most Important Stakeholder: The Patient
This session offers guidance on innovative patient-centric approaches to packaging design.
Outlining the importance of primary packaging and the need for patient involvement How involving patients in early development can help improve compliance and product durability from the design process Exploring current collaborative methods with patients to help improve current packaging standards -
Webinar De-Risking of Pharmaceutical Operations: Innovative Vial and Packaging Solutions
Gerresheimer is a system solution provider with a unique portfolio of pharmaceutical packaging solutions including drug delivery systems. High product quality in standardized format are key factors to enable an efficient and reliable fill & finish process. Gerresheimer offers a comprehensive vial portfolio to address this points: high quality Gx® Elite and Velocity® Vials. Securing the vial quality with superior dimensional consistency combined with reduced particle generation are some of the customer benefits of Gx® Elite and Velocity® Vials. EZ-fill SmartTM: introduction of this enhanced RTF packaging platform with superior features like low particles and optimized packaging to address processing and sustainability needs -
Video Corporate Image Video
This video shows how we are transforming our company into a growth asset as an innovatins leader and solutions provider. -
Webinar On the Road to Decarbonisation - Gerresheimer Moulded Glass
The presentation will be about the Gerresheimer – roadmap to decarbonization. The speaker will cover supplier-specific approaches like the furnace technology. Furthermore, Value-chain-comprising approaches in Ph-business (eg eco-design development) will be explained. In the end, success stories about joint value-chain comprising decarbonisation will be told. This session was broadcast as part of the Pharmapack show. -
Webinar Taking Secondary Packaging for Vials to the Next Level
Innovative Ready-to-Use Vial Platform for the Pharmaceutical Industry. Gerresheimer AG and Stevanato Group are addressing rising demand for RTU vials in the market and the partnership serves as a market enabler to fully support customers’ evolving needs and establish a gold standard in the industrial filling process. The new RTU solution platform will share the same secondary packaging, production process and sterilization method. A hallmark of the new RTU platform is a significant reduction in particles, improving the overall quality and performance of the RTU solutions.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






.jpg)






.jpg)


.jpg)










.png)




-file151694.png)
-file145614.png)
-file145490.png)
%20(1)-file145492.png)
-file145495.png)
%20(1)-file145502.png)
-file145504.png)
-file145505.png)
-file145507.png)
%20(2)-file145508.png)


-file143116.png)
-file142999.png)
-file143000.png)



