Rigid Films
Product Description
Südpack Medica

-
CH
-
2021On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Südpack Medica

-
CH
-
2021On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Südpack Medica (5)
-
Product Flexible Films
Our flexible films are provided with their specific properties according to their coextrusion and lamination processes. Typically, the material structures are designated for highly automated processes. Most films can both be used as base material and lidding material. -
Product Lidding
Whether from the reel, as die-cut lids, or specific defined applications, our lidding solutions provide optimal protection to maintain the sterile barrier with maximum quality under certified cleanrooms conditions is guaranteed. In addition, most of our laminated flexible films can be used as non-porou... -
Product PharmaGuard
SÜDPACK Medica is setting standards in terms of both performance and sustainability. The PP-based top and bottom webs are manufactured in a unique coextrusion process and are perfectly coordinated, particularly when it comes to their sealing performance. This guarantees not only the highest packaging quali... -
Product Pouches & Bags
With state-of-the-art lines that permits production of different primary materials converted in a format that suits the application for sterile barrier systems, a wide diversity of medical pouches with maximum quality under certified cleanroom conditions is guaranteed.
Ability to guarantee wide ... -
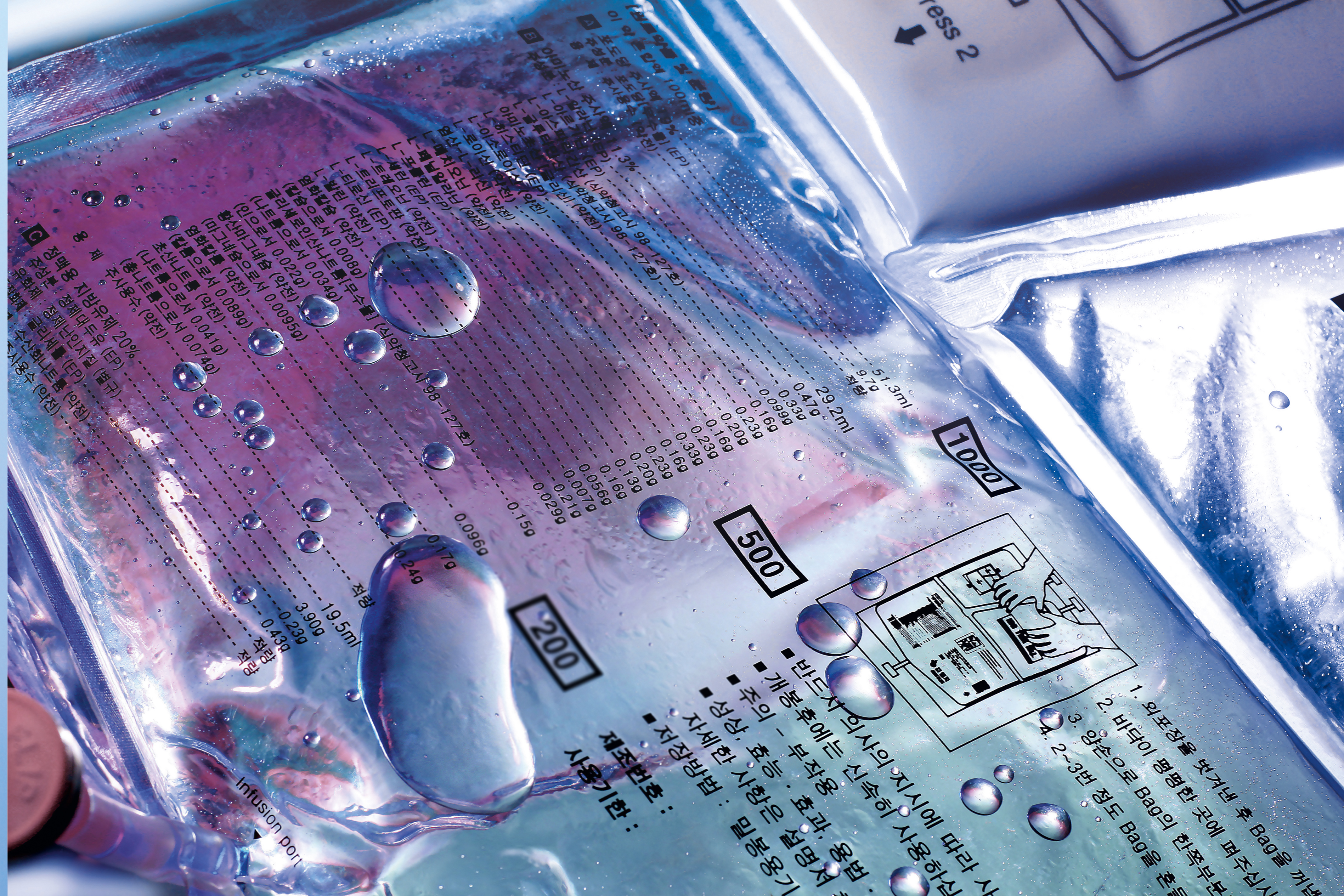
Product Rigid Films
Our thermoformable rigid films are ideal for many applications. Think about trays or blisters for surgical kits and medical devices. The films can be equipped with a variety of functionalities and barriers to properly secure its content, ensuring maximum product protection and patient safety.
Südpack Medica resources (10)
-
Webinar Recycle Ready PP Solution for Primary Pharma Packaging, PharmaGuard®
SÜDPACK MEDICA’s PharmaGuard® is recognized as one of the leading polyolefin blister packaging solutions, rewarded with the Worldstar Global Packaging Award 2024 in the Medical and Pharmaceutical category, the Swiss Packaging Award 2024, and the ‘Prestigious German Packaging Award’ in the sustainability category in 2023. After its launch early 2023, PharmaGuard® has gained lots of market interest, and is recognized as the future leading recycle ready solution. What makes the polypropylene solution unique is that it provides excellent transparency, good and stable shrinkage behavior, wider thermoforming- and sealing window than traditional polypropylene. Together with its controllable push-through lidding, the full recycle ready mono-package solution provides a child resistant and senior friendly unpackaging experience. With this, PharmaGuard® is setting new industry standards. Our speakers will take the audience on the successful journey to create a meaningful sustainable future in Primary Pharma Packaging for Oral Solid Dosed medicines. -
Video Form-Fill-Protect-Comply With Pharmaguard®
SÜDPACK MEDICA’s PharmaGuard® is recognized as one of the leading polyolefin blister packaging solutions, rewarded with the Worldstar Global Packaging Award 2024 in the Medical and Pharmaceutical category, and the ‘Prestigious German Packaging Award’ (Deutscher Verpackungspreis) in the sustainability category in 2023. After its launch early 2023, PharmaGuard® has gained lots of market interest, and is recognized as the leading recycle ready solution for the future. What makes the polypropylene solution unique is that it provides excellent transparency, good and stable shrinkage behavior, wider thermoforming- and sealing window then traditional polypropylene. Together with its controllable push-through lidding, the full recycle ready mono-package solution provides a child resistant and senior friendly unpackaging experience. With this, PharmaGuard® is setting new industry standards. Our speaker will take the audience on the successful journey to create a meaningful sustainable future in Primary Pharma Packaging for Oral Solid Dosed medicines. -
Webinar First Choice PP for Primary Pharma Packaging
The pharmaceutical industry is in a transformation-phase, trying to move from linear to circular economy models. PharmaGuard is recognized as one of the leading polyolefin blister packaging solution, and recently has been rewarded with the ‘Prestigious German Packaging Award’ (Deutscher Verpackungspreis) in the sustainability category. After its launch early 2023, PharmaGuard has gained lots of market interest, and is recognized as the leading recycle ready solution for the future. With its excellent transparency, good and stable shrinkage behavior, and a wider thermoforming- and sealing window, together with its controllable push-through lidding, PharmaGuard is setting new industry standards. You will learn more about: PharmaGuards’ different segment specific solutions, e.g. for applications with higher barrier demands. The environment footprint of different solutions. How the pharma industry can significantly reduce carbon emissions in a safe and efficient way. -
Webinar Keynote Address: Setting the Scene for Sustainability in Pharma
Keynote Speaker: The importance of embedding sustainability within your overall organizational strategy Companies need to understand the long-term value of sustainability, not only to ensure a positive impact on society and the environment, but also to grow their businesses -
Webinar Unlocking Value: Paving the Path of a Committed Value Chain with PP
Plastic solutions in healthcare are vital. Though carbon emissions and wastefulness are inherent in today’s linear economy, changes are needed across the pharma and healthcare industry. To manage this in a safe way, strong value-chain collaboration is needed.
Key talking points include:
Assessing Borealis Bornewables™ and Borcycle™ C solutions in existing polypropylene medical grades Bormed™ Analysing how SÜDPACK PharmaGuard™ can be a great mono-PP solution for blisters and why it can help the pharma industry to materialize significant reduction in carbon emissions more efficiently. Reviewing case studies in recycled and renewable feedstock and why there are no changes needed in current regulations -
Webinar Decarbonising Chemical Supply Chains with the Product Carbon Footprint Guideline
With a science-based target set for 2030, Merck has launched a Supplier Decarbonization Program to lower the greenhouse gas emissions along its supply chain. Creating transparency of the emissions associated with the products Merck purchases is key to understanding how this reduction is progressing. Companies can demonstrate reductions of this typically large share of their emissions profile by collecting product carbon footprints (PCF) from their suppliers.
Whereas many companies have begun to assess their GHG emissions, few are at the stage of routinely calculating PCFs according to standardized methodologies. The PCF Guideline developed by Together for Sustainability addresses this challenge for molecules used in the chemical, pharmaceutical and other industries and provides tailored instructions on how to perform these calculations in compliance with existing guidelines like ISO or GHG protocol. This collaborative publication moves corporations towards exchanging harmonized and reliable data that support tangible GHG emission reductions -
Webinar Promoting Circularity in Packaging: The Sustainable Packaging Model from Ferrer
Ferrar’s objective is to lead change towards more sustainable production by adopting criteria and commitments that minimize the environmental footprint of its containers and packaging
For this, it has designed the Sustainable Packaging Model, with the participation of Anthesis Lavola.
The Model has 4 strategic axes and each one has specific and monitorable objectives to achieve them: Design for Good Natural resources protection Zero Waste loop and Collaboration In this case study session, Ferrer and Anthesis Lavola will deep-dive into the Sustainable Packaging Model, an initiative that can serve as inspiration for those looking to progress towards packaging circularity -
Webinar Putting ESG into Practice – Creating Responsible Supply Chains via Common Audit Approaches
The complexity and risks found within the global pharma supply chain cannot be understated and key stakeholders from customers to regulators, and investors are increasingly expecting the industry to both manage ESG risks and embed sustainability throughout the value chain.
This can only be achieved through industry-wide collaboration that establishes shared frameworks for what a responsible supply chain looks like. The Pharmaceutical Supply Chain Initiative (PSCI) and our membership of 70+ pharma and healthcare companies are leading this collaboration.
Through our Shared Audit Program, we are putting ESG into practice and building responsible supply chains. -
Webinar Panel: How Pharma Can Build a Meaningful Circular Economy
Join the discussion with our thought leaders from across pharma to discuss what’s next for the sector in terms of sustainability and the logistics of creating a circular economy.
Building a circular economy through collaboration
Setting targets and defining your sustainability strategy
Sharing current practices in packaging, process chemicals, and manufacturing to improve other sectors" -
Webinar Sustainable Primary Pharma Packaging and Benefits of PP
For the past 20 years in healthcare, Polypropylene (PP) has been the most utilized plastic across a range of applications, from high-risk applications such as IV parenteral packaging to medical devices and diagnostics. PP offers alternatives to glass, metal and other plastics, such as PS or PVC. The pressure to find more environmentally friendly packaging solutions has increased significantly. SÜDPACK Medica’s Ecoterm™ Pharma PP pushes thermoforming of PP systems to a new level in terms of barrier level and shrinkage control. The change from PVC now becomes much easier. Halogen-free alternatives are a key consideration for making these sustainable decisions. Our expert speaker will talk about the ideal blister packaging and the reason why PP is the recommended material for oral solid dose packaging. You will hear more about PP as a polymer, advantages, focus areas, and recent LCA studies including the topic of sustainability.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-file142926.png)




-file142934.png)












-comp267646.png)



















































.jpg)