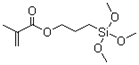
Rabies Vaccine(Vero cell)for Human Use

Product Description
Olympic Star Pharmaceutical Co Ltd

-
CN
-
2015On CPHI since
Company types
Categories
Specifications
Olympic Star Pharmaceutical Co Ltd

-
CN
-
2015On CPHI since
Company types
More Products from Olympic Star Pharmaceutical Co Ltd (21)
-
Product Human Immunoglobulin for Intravenous Injection
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes human immunoglobulin for intravenous injection. It belongs to blood product category. Contact us for more information. -
Product Human Rabies Immunoglobulin
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes human rabies immunoglobulin. It belongs to blood product category. Contact us for more information. -
Product Human Tetanus Immunoglobulin
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes human tetanus immunoglobulin. It belongs to blood product category. Contact us for more information. -
Product Irinotecan hcl
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes irinotecan hcl. It belongs to active pharmaceutical ingredient category. Contact us for more information. -
Product Paclitaxel
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes paclitaxel. It belongs to active pharmaceutical ingredient category. Contact us for more information. -
Product Pipothiazine palmitate
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes pipothiazine palmitate. It belongs to active pharmaceutical ingredient category. Contact us for more information. -
Product Recombinant Human TNF Receptor-Ig Fusion Protein for Injection
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes recombinant human tnf receptor-ig fusion protein for injection. It belongs to monoclonal antibody category. Contact us for more information. -
Product Varicella Vaccin live, freeze-dried
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes varicella vaccin live, freeze-dried. It belongs to vaccine category. Contact us for more information. -
Product Adenosine injection
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes adenosine injection. It belongs to bio-products category. Contact us for more information. -
Product Cabazitaxel
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes cabazitaxel. It belongs to active pharmaceutical ingredient category. Contact us for more information. -
Product Cladribine
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes cladribine. It belongs to active pharmaceutical ingredient category. Contact us for more information. -
Product Docetaxel
Olympic Star Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes docetaxel. It belongs to active pharmaceutical ingredient category. Contact us for more information.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance