- Home
- Sarjen Systems Private Limited
- Quality - QEdge - Enterprise Quality Management Suite
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Quality - QEdge - Enterprise Quality Management Suite
Product Description
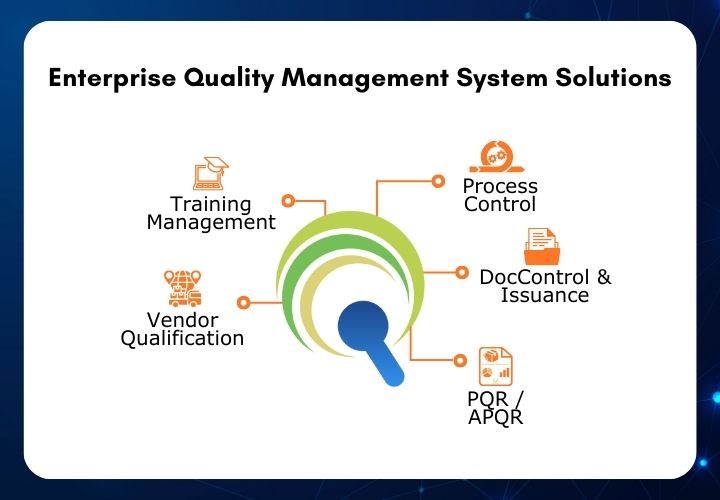
Sarjen Systems’ QEdge is an enterprise-wide Quality
Management System (QMS) specifically designed for pharmaceutical manufacturing.
This risk-based QMS offers a comprehensive suite of solutions, including:
- Process Control
- Document Control & Issuance
- Training Management System
- Vendor Qualification Management
- Product Quality Review (PQR)
- Audit Management System
- Trend Analysis
- New Product Introduction
- Artwork Management System
- User Access Management
What makes QEdge different: Configurable & Automated Workflows | Process Versioning | Automated Intra-Module Dataflow | Field & Functional Audit Trails | Power BI Dashboards | TPA Integration
Compliance: GxP, FDA 21 CFR Part 11 and Part 820, EudraLex Annex 11, GAMP 5, ISO 9001:2015, GDPR
Sarjen Systems Private Limited

-
IN
-
2019On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Consultancy
IT/Software solution provider
Primary activities
Clinical Research
Contract Research Organisation
Other
Regulatory Affairs
Technology
Contact info
-
-
-
6th Floor, Arista, 100 Feet Anand Nagar Rd, Near Rahul Tower Cross Road., Prahlad Nagar, 380015, Ahmedabad, Gujarat, India
Categories
- Biopharmaceutical Products Technology and Software (Biopharmaceutical Products)
- Biopharmaceutical Services Technology and Software (Biopharmaceutical Services)
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) API Contract Manufacturing
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Computer software
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Consulting Services
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Contract Manufacturing of Dosage Form Drugs
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Contract Manufacturing/Exclusive Synthesis of Fine Chemicals
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Custom Manufacturing (general category)
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Data services
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Development & Validation of Analytical Methods
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Drug Discovery
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) E-Business
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Formulation development
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Marketing Services
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Process Optimisation
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Regulatory Affairs
- Pharmaceutical Machinery & Technology (Products) Health & Safety Products
- Pharmaceutical Machinery & Technology (Products) Medical Devices
- Pharmaceutical Machinery & Technology (Products) Pharmaceutical Technology (general category)
- Pharmaceutical Machinery & Technology (Products) Process Automation & Controls
- Pharmaceutical Machinery & Technology (Products) Validation
Specifications
- Details450k+ Training Records Generated | 5000+ SOP’s Implemented | 200k+Quality Processes Executed | 5000+ Vendors Qualified | 40+ Regulatory Audits | 15+ Country Reach | 75+ Implementations
- ModelSaaS, Pre-Validated, On-Premises, Cloud Ready, Web-based, Industry Ready
- Supplied fromIndia
Sales Markets
- Middle East Region (e.g. UAE)
- Oceania
- North America (USA, Canada)
- Africa
- Central America (e.g. Mexico)
- East Asia (e.g. China, Japan, Korea)
- Europe - EU countries
- Europe - non EU (e.g. UK, Russia, ex-CIS countries)
- South America (e.g. Brazil, Colombia)
- South Asia (e.g. India, Pakistan, Sri Lanka)
- South East Asia (e.g. Thailand, Philippines, Singapore)
Sarjen Systems Private Limited

-
IN
-
2019On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Consultancy
IT/Software solution provider
Primary activities
Clinical Research
Contract Research Organisation
Other
Regulatory Affairs
Technology
Contact info
-
-
-
6th Floor, Arista, 100 Feet Anand Nagar Rd, Near Rahul Tower Cross Road., Prahlad Nagar, 380015, Ahmedabad, Gujarat, India
More Products from Sarjen Systems Private Limited (2)
-
Product Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
AI-enabled, cloud-ready,360 pharmacovigilance solution for safety database management. An end-to-end globally compliant safety software for Drugs, Devices, Vaccines and Combinational products from case intake to submissions and reporting. • Case Intake • Case Processing • Case Submission • Risk Manage... -
Product Regulatory - KnowledgeNET - RIMS | eCTD | eCSV
KnowledgeNET is a global dossier publishing & management software empowering pharma industries to achieve regulatory compliance efficiently.
• eCTD Publishing: DMF | ASMF | CEP | ANDA | IND | BLA | PADER | PSUR | 510(K) • CTD/ ACTD and Regional Submissions • Query and Life Cycle Management ...
Sarjen Systems Private Limited resources (1)
Recommended Products
-
Product Pharmaceutical drug substances (APIs)
Seratec specializes in fine organic chemistry and is dedicated to active pharmaceutical ingredient (API) development & production for the pharmaceutical industry.
Seratec operates in highly regulated markets and where high quality products are critical.
Seratec offers a complete range of cust...
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Semi-Automatic Pilot Assembly Machine
This versatile semi-automatic assembly machine is designed for efficient production of small to medium-sized batches, making it ideal for clinical testing and pre-industrialization phases. It can also handle complex-shaped parts introduction or low volume production. With a robust capacity of 700 pieces pe...
-
Product ALLERGOFF® allergen-neutralizing spray
ALLERGOFF® spray is an innovative solution for neutralization of house dust allergens. It is designed to protect against harmful impact of allergens present in house dust, such as: house dust mites allergens, pollen, pet and fungal allergens. It neutralises house dust allergens by changing their chemical ...
-
Product UV Light Blocking Tyvek Equipment Covers
UV Light Blocking Tyvek Equipment Covers are designed to keep cleaned pharmaceutical equipment and supplies clean and protected from external sources of contamination while also providing in-process protection to light sensitive biotech products.
-
Product Centrifuge Liners & Bags
Sefar products for centrifugal filters are matched to the filter machines used, whether they are band centrifuges, bag centrifuges or inverting centrifuges. Our ready-made solutions are based on optimized Sefar designs that have been proven in use over many years.
Thanks to our wide range of fab...
-
Product MMAE
A GMP quality payload for your drug-conjugateprogram• Samples available free of charge• Validated analytical methods• Free licensing and no royalty payments
-
Product GENiSYS Lab
AST’s GENiSYS Lab bench top systems are ideal for drug product development, process development, and small batch cGMP production applications. Each system is designed to automate the critical aseptic operations for vial, syringe and cartridge processing to reduce contamination risk and product variability....
-
Product Custom Protein Synthesis
We provide a Custom Protein Synthesis Service, using a chemical method that synthesises proteins amino acid by amino acid and making modifications on an atomic-scale. We work closely with our partners in designing, customising and optimising the proteins that is synthesized in an automa...
-
Product Life Science: Computer System Validation
Validation of computerized systems (CSV) according to GxP Guidelines has been a basic requirement in the life science industry for many years now. However, as the complexity of the IT landscape and the degree of automation has increased, the effort required to keep compliance has risen as well. Of cour...
-
Product Analytical Development:
•Development and validation of analytical methods to ensure accurate quantification, purity assessment, and impurity profiling.
•Characterization of raw materials, intermediates, and final APIs using techniques such as HPLC, GC, MS, NMR, and XRPD.
•Stability studies and fo...
-
Product CordenPharma ONE PARTNER CDMO Video
Your ONE PARTNER CDMO for Fully-Integrated Supply
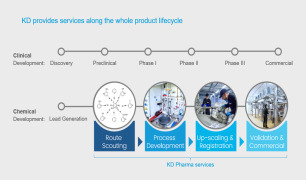
As your ONE PARTNER CDMO, CordenPharma provides contract manufacturing of APIs, Excipients, Drug Products & Packaging in an integrated solution spanning all product life cycle stages, from preclinical to commercial, supported by dedicated regulator...
-
Product GMP or non-GMP Contract Manufacturing
Actylis continues to expand its manufacturing capabilities by serving customers on a contract basis for exclusive manufacture of fine chemical compounds. We can provide a comprehensive tailor-made service to our clients, ensuring process development, optimization and commercial scale-up of processes are ca...
-
Product IT/EU REGULATORY AFFAIRS - Support and management of Technical documenta...
OP Pharma has been providing innovative services in the Regulatory Affairs field for more than 20 years to small to big pharma Companies in Italy as well as in the European Union.
Our expertise covers the lifecycle management of pharmaceutical products, whole or in part, ranging from digitization ...
-
Product High-potent Dosage Forms
We have the experience, expertise, and facilities at Sever Pharma Solutions to safely work with your high-potent substances. This includes manufacturing products containing APIs that are ranked in the highest occupational exposure bands (OEB) up to and including OEB6.
Our focus on safety starts ...
-
Product Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
AI-enabled, cloud-ready,360 pharmacovigilance solution for safety database management. An end-to-end globally compliant safety software for Drugs, Devices, Vaccines and Combinational products from case intake to submissions and reporting. • Case Intake • Case Processing • Case Submission • Risk Manage...
-
Product CDMO Services
Genovior is a dedicated CMO/CDMO, focusing on providing clients excellent, efficient, one stop services and solutions, from clinical trials (IND/NDA) to commercialization.
Our services include:
1. Process Development
2. Formulation Development
3. Analytical Development
...
-
Product Computer System Validation (CSV) Solutions
ProPharma's validation professionals leverage the latest risk-based Computer System Validation (CSV) and Computer Software Assurance (CSA) techniques to ensure that our clients' systems are ready for inspections from the FDA, EMA, MHRA, and other regulatory agencies. Our consultants have extensive experien...
-
Product CONTRACT MANUFACTURING - SMALL MOLECULES
KD Pharma’s professional expertise, state-of-the-art assets and proven track record in regulatory compliance allow us to become your preferred CDMO solutions partner.
Your Agile Experts
• Responsive to our customers’ needs in a more dynamic customer-centric way • Adaptive to the most diverse re...
-
Product Pediatric Development
Pediatric formulation and product development
The development of acceptable, palatable pediatric formulations is a key feature within the industry today, driven by patient needs and regulatory requirements. Quotient Sciences has extensive knowledge and capabilities that enable us to provide you...
-
Product Lozenges
Our range of user-friendly dosage forms includes lozenges. Lozenges are an easy way to take medication and, with a wide range of flavorings available, there is something for everyone. Designed to dissolve or disintegrate slowly in the mouth, lozenges are capable of providing both a localized effect and a...
-
Product E business and Media communications
Contract Pharma LLC is a multi-marketing media company offering a magazine, Enewsletters, a website and an annual conference (Sept 26-27, 2019) Now in it's 20th year, Contract Pharma is the leading publisher serving this sector. We carry the lion's share of all advertising in the contract ...
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product Rosemary Extract
• Name- Rosemary Extract • Specifications- Rosmarinic Acid 20% & 40% by HPLC, \Ursolic acid 20% & 30% by HPLC, Carnosic acid 5% , 10% & 20% by HPLC • Application- Anti-oxidants & Anti-inflammatory • Botanical Source- Rosmarinus Officinalis To know more, ple...
-
Product Oradel
The proprietary Oradel platform is a line of oral delivery coating techniques that can be used individually or together to meet any custom delivery requirements. Our Wurster coating capabilities allow us to add numerous coating layers to a core. These layers could be a simple coating formulation or could c...
-
Product Pre-mixed IV solutions: vials and bottles
Glass vials (5mL-50mL) Glass bottles (50mL-500mL)
Grifols Partnership is specialized in developing and manufacturing high quality sterile solutions in glass vials and bottles to suit every need in both the human and veterinary markets.
-
Product Research & Development of medical devices
We provide a complete and comprehensive range of services from A to Z for substance-based medical devices: from product idea, through development and research, certification, registration and product delivery to the customer.
With us, you can easily launch a new substance-based ...
-
Product Biorela® Total
Biorela® Total is a powerful polybiotic and a member of the strongest family of probiotics – Biorela®. The company Milsing has been developing strong products with clinically tested combinations of probiotic bacteria within the Biorela® line for 13 years. Biorela® Total is the latest addition to the portfo...
-
Product Medical & Regulatory Services - Inhaled Products
Our formulation, device and development services are supported by our medical, regulatory, device vigilance and pharmacovigilance teams to safeguard your programme and ensure the smoothest path to product approval.
Our in-house team can support with:
Global regulatory development strategy...
-
Product Data Integrity Assurance
Data is a fundamental part of any Life Science production cycle and has become a major concern for global regulatory authorities.Choose PQE and ensure patient safety and business continuity within the entire product life cycle.
-
Product OTF - Oral Thin Films
Oral thin films are loaded with active substances. The Thin films are taken orally and dissolve immediately in the mouth or are applied to the mucosa. For transmucosal films, the active substance enters the bloodstream directly via the oral mucosa, without having to first pass through the gastrointestinal ...
-
Product PURO EPITHEL - Carbomer + Dexpanthenol (Ophthalmology - Dry Eye)
Specifically formulated to offer effective symptoms relief for patients suffering from dry eye syndrome and to stimulate epithelial corneal reparation in case of corneal erosion, burns, after ocular surgery (cataract and refractive) and in prolonged use of contact lenses .
Ophthalmic gel...-comp246544.jpg)
-
Product Oral Solid Dosage Form Contract Manufacturing
Pfizer CentreOne excels in the manufacture of oral solid dosage forms.
From hormones to immunosuppressants to sensitizers, we're known for our:
• Specialized technologies • Containment expertise • Deep technical and commercial know-how You can count on our devotion to you and your drug, ...
-
Product Development Oral Solid Dosage forms
XEDEV provides wide range of technologies going from powder to tablet in order to develop your final oral solid dosage form. • spray-drying • encapsulation • granulation (wet, dry and hot) (batch and continuous) • tableting • coating Contact us for more information.
-
Product Ginhyal Deli - intimate gel against dryness
Non-hormonal gel based on a patented moisturizing and restructuring compound and hyaluronic acid. Its delicate formulation counteracts vulvo-vaginal dryness and its linked discomfort. It acts as an intimate lubricant and restores intimate mucosa, promoting its natural firmness and elasticity. It is availa...
-
Product F2CS - "Fast to Clinical Supply" Platform
Your innovative therapy is ready to reach a key milestone with the IND date quickly approaching. You need a reliable partner to formulate and manufacture your therapy to meet the IND filing requirements and the dose needs for phase I studies fast.
Every innovation is unique so your partner needs...
-
Product Development
Synerlab provides wide range of services which includes development. Contact us for more information.
-
Product Development Service
Lomapharm GmbH provides wide range of services which includes development service. It provides the whole range of control services - from raw material testing to stability testing according to GMP and ICH regulations. One of the main areas of competence and experience is also available for galenic dev...
-
Product Finished Product Manufacturing Services
Midas Pharma offers a wide range of pharmaceutical services which includes finished product manufacturing services. Midas offers a complete package - from API sourcing and technology transfer to variation filing - or just the modular services you require.This includes: • identification and qualif...
-
Product API/Formulation Manufacturing Services
We have access to the manufacturing facilities of Dr. Reddy’s and a network of strategic business partners. Our pharmaceutical drug manufacturing services offer a wide range of manufacturing services from a few kilos to multi tons of cGMP manufacturing of Key Starting Material (KSM) / Registered ...
-
Product SLIMStat - Shelf-Life Projection Software
SLIMStat is extremely easy-to-use software developed and validated specifically to determine the shelf life of drug products that have been placed on stability. This interactive and intuitive tool empowers non-statisticians the ability to generate statistical analyses with complete confidence. Fully v...
-
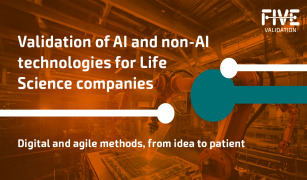
Product AI and non-AI validation technologies for Life Science companies
We specialize in IA and non-AI Computer System Validation, equipment validation, utilities qualification, and IT/OT infrastructure qualification. Our services comply with the regulatory standards of the EMA, FDA, WHO, and other key Life Science authorities across Europe and the Americas. With a proven trac...
-
Product PeP11 LabCal Excel Platform
PeP11 LabCal offers a Single Validation SaaS Solution Platform that manages all Excel spreadsheets, eliminating the need for individual validations. This comprehensive electronic Excel solution features an Electronic Signature, Audit Trail, and Role-Based Access, rendering paper signatures unnecessary ...
-
Product Complex drug formulation
We like to get involved right from the start of a development project and work closely together with our clients and partners on transforming the idea into a product with a solid and commercially viable target product profile (TPP). We set the bar high but always work step by step with a clear focus on...
-
Product Expert witness and intellectual property support
Intertek offers wide range of pharmaceutical services which includes expert witness and intellectual property support for pharmaceuticals. It belongs to pharmaceutical and healthcare consulting services category. It includes expert witness and intellectual property support services, intellectual property s...
-
Product Food Supplement range of products
We offer a wide range of food supplements ready to be put on the market. Available formulations in Basic Health - Energy - Osteo and Joint Care - Immuno and Womens' Health.
We can also realize your idea of product and offer a full customised development.
Contact us for more info
-
Product Cortellis Drug Discovery Intelligence™
Biology, chemistry and pharmacology data -all in one place. Accelerate drug development with early, comprehensive intelligence. Collating the necessary data to identify the likelihood of a drug's success as early as possible requires considerable resources. Cortellis Drug Discovery Intelligence(TM) ...
-
Product Development and engineering services by GEMÜ
End-to-End Solutions for Cleanroom Plastic ManufacturingGEMÜ is your trusted partner for cleanroom plastic manufacturing, offering comprehensive development and engineering services tailored to the needs of medical technology, pharmaceutical packaging, and other highly regulated industries. We collaborate ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance