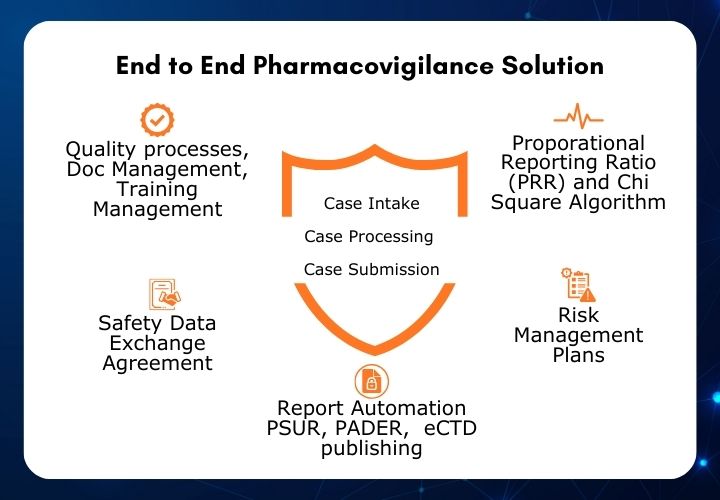
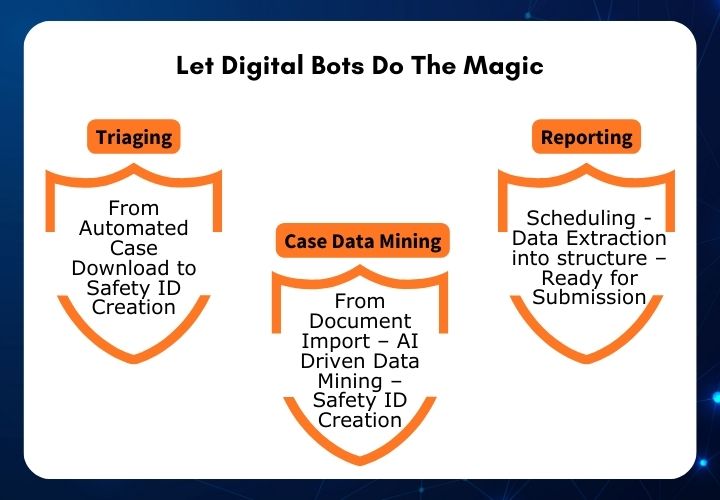
Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
Product Description
Sarjen Systems Private Limited

-
IN
-
2019On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Sarjen Systems Private Limited

-
IN
-
2019On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Sarjen Systems Private Limited (2)
-
Product Regulatory - KnowledgeNET - RIMS | eCTD | eCSV
KnowledgeNET is a global dossier publishing & management software empowering pharma industries to achieve regulatory compliance efficiently.
• eCTD Publishing: DMF | ASMF | CEP | ANDA | IND | BLA | PADER | PSUR | 510(K) • CTD/ ACTD and Regional Submissions • Query and Life Cycle Management ... -
Product Quality - QEdge - Enterprise Quality Management Suite
Sarjen Systems’ QEdge is an enterprise-wide Quality Management System (QMS) specifically designed for pharmaceutical manufacturing. This risk-based QMS offers a comprehensive suite of solutions, including:
• Process Control • Document Control & Issuance • Training Management System • Ve...
Sarjen Systems Private Limited resources (1)
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance