- Home
- ZAHORANSKY Automation & Molds GmbH
- PRIMA Z vial
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
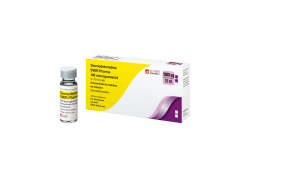
PRIMA Z vial
Product Description
PRIMA Z vial - Cost savings through continuous aseptic production of plastic vaccine containers
A medication container for a liquid vaccine can use plastic instead of glass thanks to ZAHORANSKY's advanced injection mold tooling and manufacturing equipment. This is accompanied - due to the elimination of the risk of breakage - by advantages in storage and handling in doctors' offices, laboratories, hospitals, as well as in emergency medical services. The special feature: In this special application, sterilization could be completely dispensed, since the product runs directly into aseptic filling after production via injection stretch blow molding - a novelty in this area.
ZAHORANSKY Automation & Molds GmbH

-
DE
-
2021On CPHI since
-
500 - 999Employees
Company types
Manufacturer/Innovator
Departments
Sales
Categories
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services) Vial/ Syringe Filling
- Contract Packaging Services Sterile Injectables Manufacturing Services
- Packaging Materials and Equipment (Products) Injectable systems and components
- Packaging Materials and Equipment (Products) Primary Packaging (Packaging Materials and Equipment (Products))
- Pharmaceutical Machinery & Technology (Products) Aseptic technology
Specifications
- DetailsContinuous aseptic production of plastic vaccine containers
- Selling PointsContinuous aseptic production
To ensure the aseptic production of this special product, the injection stretch blow molding tool and the assembly unit work together in a class 6 clean room cell. The vial is removed directly from the injection molding chamber and sealed without leaving the sterile environment.In the next steps, the pre-assembled container is ejected from the system, transported to the filling system via a transport unit, filled and finally fed to the packaging machine.The continuous aseptic production of the main components eliminates the washing, drying, testing and sterilization processes otherwise required for glass, resulting in considerable cost savings.
ZAHORANSKY Automation & Molds GmbH

-
DE
-
2021On CPHI since
-
500 - 999Employees
Company types
Manufacturer/Innovator
Departments
Sales
More Products from ZAHORANSKY Automation & Molds GmbH (1)
-
Product PRIMA Z syringe
Production lines for any requirement
Based on customer-specific product designs, a cycle time of 15 to 17 seconds can be achieved on the three currently available PRIMA Z production lines using the no-human-touch process. Ready-to-use syringes (Pre-Filled Syringes, PFS) according to ISO11040-6 w...
Frequently Viewed Together
-
Product high capacity aseptic filling - bp 460
When it comes to high capacity production of SVP containers at the lowest unit costs, our machine type bp 460 is the first choice. Regulatory bodies consider BFS as advanced aseptic manufacturing – only one operator is required to supervise the process. Multiple containers are formed, filled and ...
-
Product Complex injectables
25+ years of experience in complex formulation development, including ready to-use specialty products and novel drug delivery systems
Proven expertise in niche and complex dosage formats (single and dual-chamber bag, Lyo-vials, PFS, cartridges) to address hospital needs and improve patient experienc...
-
Product Glass Vials
From 1 ml to 30 ml glass vials. Type I, II and III glass, depending on your needs Amber and clear colours Normal, siliconized or ready for lyophilization Prewashed, in RTU packaging and nesting All in ISO standard measurements Capacity to develop according to client's specifications
-
Product Sterile Pharmaceuticals
Lomapharm GmbH provides wide range of services which includes sterile pharmaceuticals. The modern clean rooms complies with the increasingly stringent specifications laid down for sterile production. The filling line fills ear-drops, eye-drops and nose-sprays, which are usually prepared by us in medium...
-
Product AT-Closed Vials® - RTF Vials for for injectable drugs, designed to be us...
The vials are particularly suitable for use with sensitive advanced therapeutical products such as cell and gene therapy preparations with special requirements for processing, transport and storage. AT-Closed Vials® are designed to be seamlessly fed into robotic filling lines for automated, aseptic fil...
-
Product Vials
Mefar ilac sanayii offers wide range of products including vial filling between 2 ml through 300 ml.
Mefar's all services have EMA certification.
Contact us for more information.
-
Product NABIQASIM INDUSTRIES / Lyophilization of PPIs / Lyophilized Infusion / I...
Lyophilization or freeze drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase. The process consists of three separate, unique, and interdependent processes; f...
-
Product Ampoules Filling and Sealing - RSF Series
The filling and sealing machines RSF are suitable for aseptic dosing of liquid products and sealing of open or closed ampoules.
As an option, RSF machines can be used for filling and rubber stoppering of vials.
Main features:
- Perfect operational repeatability;- Comp...
-
Product DecFill® Aseptic Filling Solutions
DecFill® solutions are configured to meet a wide range of product and customer requirements. These include de-bagging, de-lidding and de-nesting units and various other options to accurately fill liquids, powders and gels into the various containers.To ensure highest process performance within a controlled...
-
Product Vanrx Liquid / Lyo Workcell System
The Vanrx Liquid / Lyo Workcell System is a robotic filling and lyophilizer loading line. It can process vials, syringes or cartridges into liquid or freeze-dried drug product formats. This Workcell System can continue to produce liquid dosages during freeze-drying cycles. The Vanrx Workcell System co...
-
Product Steam sterilisation chevron peel pouch with steam indicator and hang hol...
Improve the prep time, performance, microbial barrier, and cleanliness of your autoclave peel pouches. Manufactured and packaged in certified ISO6 Cleanroom Integrated Hang Hole is ideal for use with Isolator VHP decontamination cycles Easy, fast, and reliable self-sealing closure system Autoclavable a...
-
Product Drug Product Fill-Finish Services
Fill-Finish Capabilities (product type) and Capacity:
Sterile liquid products
Biosafety level (BSL) 1, BSL 2 (OEL >= 10 µg/m³)
Vaccines (inactivated)
Current capacity: ~40M units/year

-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Filling: Ampoules, vials, syringes, pouches, bottles or blisters
Our core competency lies in customised batch production supported by product formulation, (aseptic) processing and filling followed by a variety of terminal sterilisation techniques as per the requirements or a complete aseptic closed manufacturing system. The following filling units are available: • 2...
-
Product Pharma Custom Automation Solution for filling and assembly of combinatio...
Comecer is specialized in the design and construction of automatic systems for the filling and assembly of innovative drug delivery devices and single use disposables. Recent demands have increased the introduction of novel drug delivery devices and single-use disposables in the marketplace. Comecer...
-
Product Dexmedetomidine EVER Pharma
Dexmedetomidine is a highly selective alpha-2 adrenergic receptor notable for its ability to provide sedation without the risk of respiratory depression.
The first Dexmedetomidine in Europe indicated for both ICU and procedural sedation.
-
Product VIJOINT HCC
Vijoint HCC is a patented fixed combination of Hyaluronic Acid (HA) sodium salt 2%, chondroitin-Sulfate 2% (CS) and Hydroxypropyl beta-Cyclodextrin 1%. HA AND CS ACT IN SYNERGY TO CONTRAST DISEASE SYMPTOMS AND SLOW DOWN ITS PROGRESSION. YOUNG TARGETHYALURONIC ACID SYSADOA: SYMPTOMATICSLOW-...
-
Product Sterile Manufacturing
Sharp Sterile offer sterile fill finish contract manufacturing. We specialize in small-scale, isolator-based sterile filling of vials, syringes, and cartridges for biotech and pharmaceutical industries. We also offer terminal steam sterilization, specialty filling, lyophilization services, analytical suppo...
-
Product Vers-A-Tech™ - Cell & Gene Therapy Processing
Vers-A-Tech™ (Versatile Aseptic Technology) is a semi-Automatic or fully automatic modular versatile platform consisting of machines that come together to processes Bags & Nested Containers. BAUSCH Advanced Technology Group launches First of its Kind Platform Vers-A-Tech™ ...
-
Product Polymer-based delivery systems
Polymer-based dosage forms continuously administer the drug to the patient for an extended period, varying from weeks to years. These controlled-release products are very suitable for specific indications, patient populations, and drugs due to the long duration of action, avoidance of first-pass metabolism...
-
Product Sterile Multidoses
As a specialist in manufacturing and aseptic filling of non-injectable sterile liquid products, with or without preservatives, Synerlab is always at the forefront of this expertise in order to anticipate market evolutions. Nowadays, it is a key player internationally in eye and eardrops, as well as nasal, ...
-
Product High Containment isolator for Micronization
The latest challenge was to design a multi-purpose isolator used to micronize down to a few microns covering a wide range of batch size and assuring a Containment Performance Target (CPT) of 50ng/m3. Inside the isolator, a 4”, 8” or 12” jet mill can be used.
To achieve this, a 9-chamber isolat...
-
Product Volumetric Dosing Pumps for Filling Machines
Made-to-measure Design – Custom Made Service - Single-block manufacture in 316L Stainless Steel or Titanium (No Weldings) - DLC PVD and CrN PVD Coating for Maximum Fluency and Maximum Hardness Surface (No Contamination of Chrome in Contact with the Final Product) - Cleaning In Place – Sterilisation In Plac...
-
Product Stoppers & Caps
Features of our Closures; • Have passed the leakproofness test and are reliable, • Can be supplied with an aluminum seal installed, • Can be produced in different colors, • Our child-proof caps provide the ideal balance between innovative design, ease of use and reliability, ...
-
Product Snowbell Vial Line (Liquid/Powder)
Complete integrated line consisting of : • Vertical rotary vial washing machine
• Sterilizing & depyrogenating tunnel
• Liquid vial filling & bunging machine with statistical / 100% check weighing under isolator
• Transfer conveyors with isolator
• Automat...
-
Product 5th Generation Prefilled Syringe
This innovative medical device is the newly patented 5th generation of prefilled syringes. Its innovative design overtakes the weak points of standard pfs, particularly by eliminating the break-loose force and any need for silicone oil.
The absence of silicone oil makes it the pe...
-
Product Capsu'Matic
An innovative range of capsules dispensers! Capsu’Matic dispenses all forms of gel capsule, one by one, with a simple press. No more shaking several into your hand and putting the extras back into the container.
-
Product BLOW FILL SEAL
Custom designed containers for pharmaceuticals, nutraceuticals, biologics, etc. all made be one single Machine - ALPS Hybrid Model 700 Series Size Range: 0.mL -2000mL
-
Product Closures
A wide range of closures, including tamper-evident caps and CRC (child-resistant closures), complement the bottles and vials. Manufactured from PE, PP, or HDPE, these closures ensure product safety and meet regulatory standards like ISO 15378 and ISO 8317.
-
Product Contract Filling
Rommelag CMO fills more than 2 million containers a day for customers all over the world. With more than 50 systems in all sorts of configurations, Rommelag CMO runs one of the world’s most cutting-edge and largest bottelpack machine fleets. And they are set up to meet stringent pharmaceutical stan...
-
Product Isolation technology
Getinge's barrier isolators create a separation of raw materials, a product or an experiment from its environment. The patented DPTE® sterile transfer systems provide the means to move material in and out of an isolator or sterile zone without breaking containment.
Getinge developed the ISOFLEX-R isol...
-
Product SEELCAP®
Our products comply with tamper-evident requirements in the pharmaceutical industry and offer increased protection against tampering by means of security perforations.Proof of authenticity and provision of security (tamper-evident)Suitability for many different packaging materials and surfacesAn integrated...
-
Product Multipurpose small batch filler
xfillR is a multipurpose machine that combines the filling and closing of very different objects such as vials, syringes, ophthalmic bottles, nasal sprays, cartridges in nests, trays and bulk – all in one.
-
Product Kaiman
The Kaiman® is a rigid, nestable container with an integrated hinged lid that can be opened on two sides. The Kaiman® provides easy nesting when empty, saving up valuabe space required for storage or return transport. Label holders are located on one of the two short sides. Permanent barcode labels provide...
-
Product DataCollector - High performance IT Infrastructure for Manufacturing
The plus10 DataCollector provides a high-performance data infrastructure for the acquisition and pre-processing of machine data from any source such as sensors, machine or robot controllers. Its simple configuration and implementation transforms machine data into Big Data and creates value through high-fre...
-
Product RoSS.FILL: Automated Filling & Filtration System
RoSS.FILL is a fully automated filling & draining system for single-use bags and bottles in different sizes and quantities. It facilitates maximum flexibility and scalability of both the filling and draining process. It is possible to attach filling racks for unlimited volumes per batch with ...
-
Product Prefilled Syringes 1ml, 2.25ml, 3ml
Zhengli offers a wide range of Prefilled syringes - PFS to meet you drug product requirements:
• Size 1.5ml, 2.25ml, 3ml • Glass Type 1 • With Needle, Luer Cone, Luer Lock, • Different Needle customization • ETO Sterilization • Round and Cut Flange • Nest of 160/100 ... -
Product Total opening drums
Total opening drums with screw lids offer maximum freedom/flexibility during filling and emptying. They provide easy and safe removal of filled liners or other valuable contents.
Plastic High Performance Packaging by CurTec is clean, safe, and secure and offers maximum protection to active ingredi...
-
Product Gel with Hyaluronic + Azelaic Acid
Effective treatment against skin impurities • Has an inflammation reducing effect • Accelerates the reduction of blackheads • Slows down the excessive growth and spread of acne bacteria • Regulates the excess production of skin oils • Normalizes the natural appearance of the skin • Reduces annoying re...
-
Product Nested container filling and stoppering solution Ultra-N serious
Capable for Nested Syringes, Cartridges and Vials Processing
Fully Automatic De-bagging, De-lidding, Filling and Stoppering, Vials De-nesting
Output Up to 15000 pcs/h
Available for Mechanical Stoppering , Vacuum Filling and Stoppering

-
Product Safer® Reverse Prefilled Syringe
The shielding system automatically activates after the injection, and the needle gets fully covered. Using just four plastic components and minimal manufacturing materials, SafeR® Reverse Prefilled Syringe is completely scalable and adaptable to the size of the existing syringe.
The desi...
-
Product GENiSYS R
GENiSYS R is an aseptic filling and closing system that solves small-batch production challenges. GENiSYS R uses flexible robotic automation to process ready-to-fill vials, syringes and cartridges. GENiSYS R offers standard modular configurations that allow the system to be tailored with isolator systems, ...
-
Product PAMIMAGE (Iopamidol Injection USP)
Iopamidol is an effective non-ionic, water-soluble contrast agent which is indicated for angiography throughout the cardiovascular system, including cerebral and peripheral arteriography, coronary arteriography and ventriculography, pediatric angiocardiography, selective visceral arteriography and aort...
-
Product AP-IS - Aseptic Processing Isolator System
THE SHORTEST WAY TO A STERILE WORLD. The AP-IS is a cGMP Class A/ISO 5 isolator system specifically designed for QC labs, pilot scale pharmaceutical production and pharmacies. It’s a flexible equipment that guarantees the best working conditions in different applications such as Sterility Testing, As...
-
Product Oral Dosing Syringe
Product Details
Size:1ml 5ml 10ml
Dosing values printed to your requirements
Fully customisable print
Suitable for pharmaceutical and veterinary applications
Scaling and printing on the pipette barrel is fully customisable and can inc...
-
Product Sterile Drug Product services
CARBOGEN AMCIS offers Sterile Drug Product services, specializing in the formulation, development, and manufacturing of injectable products. With capabilities in lyophilization, sterile filling, and handling highly potent APIs, the company ensures aseptic processing for clinical and commercial supply. ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















.jpg)