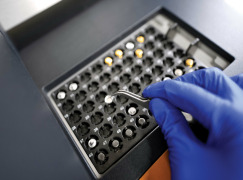
Prefilled Syringe
Product Description
AbbVie CMO

-
US
-
2015On CPHI since
-
5000+Employees
Company types
Primary activities
Specifications
AbbVie CMO

-
US
-
2015On CPHI since
-
5000+Employees
Company types
Primary activities
More Products from AbbVie CMO (13)
-
Product Drug Product
Whether you’re developing a new chemical entity or a first-to-market generic, precise dosing and drug delivery is vital for success. Regulatory agencies and clinicians want to see well-characterized, reliable doses with good bioavailability. Patients/consumers often favor a controlled release dosage form, ... -
Product Hot Melt Extrusion (HME)
Hot melt extrusion (HME) is a proven technology for bioavailability enhancement of poorly soluble API's. Unlike other formulation options, hot melt extrusion technology is solvent-free. By drawing on continuous processing, it is also more economical and can be used for abuse deference and taste masking app... -
Product Fermentation
Fermentation is a critical step in both drug and toll manufacturing, as companies look to produce the core compounds that form their eventual products. While it is possible to undertake this in-house, the expertise contract fermentation services provide can improve the quality, affordability, speed and sca... -
Product Biologics Development and Manufacturing
With patients’ lives at the end of the pipeline, biologics contract manufacturing needs to be safe, reliable and be consistent in supply. The developers of these large molecule drugs, however, are also operating in a highly competitive space. Speed-to-market is imperative, along with efficient processes an... -
Product Antibody Drug Conjugates (ADC)
hen selecting AbbVie Contract Manufacturing, you are partnering with a leading developer and manufacturer focused on accelerating and mitigating risks to program timelines and on efficiently fast-tracking your program to completion. AbbVie’s mAb and ADC state-of-the-art facility and expert scientific team ... -
Product Potent
High potency manufacturing is an expanding field with a unique set of challenges. Safety remains a primary concern for those handling cytotoxic drug products, as well as the clinicians and patients down the line. Beyond safety, pharmaceutical companies need to plan ahead for how they will manufacture high ... -
Product Erythromycin Base
For more information contact AbbVie Contract Manufacturing -
Product Erythromycin Ethylsuccinate
For more information contact AbbVie Contract Manufacturing -
Product Erythromycin Stearate
For more information contact AbbVie Contract Manufacturing -
Product Erythromycin Thiocyanate
For more information contact AbbVie Contract Manufacturing. -
Product Cyclosporine
For more information please contact AbbVie Contract Manufacturing. -
Product Aseptic Fill Finish
We offer two convenient fill-finish options for parenteral drug products: prefilled syringe and vials. As a leading pharmaceutical partner, we can provide all necessary elements of delivering high-quality parenteral products for clinical and commercial needs.
AbbVie will leverage its know...
AbbVie CMO resources (6)
-
News AbbVie gains FDA approval for latest treatment for Parkinson's Disease
AbbVie, the speciality biopharmaceutical company has once again made headway in the treatment of Parkinson’s disease. -
Brochure AbbVie CMO Services
AbbVie’s Contract Manufacturing business has been serving our partners for more than 40 years. Our contract development and manufacturing capabilities span across 11 production facilities in North America and Europe. Our capabilities are: Aseptic Fill Finish (Vial and Prefilled Syringe) Custom & Bulk API Manufacturing Oral Solid Dose Fermentation Hot Melt Extrusion Potent Biologics Packaging -
News How tech transfer and process optimisation can help patients – CPHI North America Interview
Following a successful few days in Philadelphia for CPHI North America, I was able to discuss some of the sessions with our expert speakers, gaining a little more insight into the key topics from the event. -
News Which 10 drugs are open to price negotiation with Medicare in the USA?
The Centres for Medicare & Medicaid Services, under the Biden administration in the USA, has released a list of the 10 drugs that will be open to price negotiations as part of the new legislation under the Inflation Reduction Act (IRA). -
News 10 Major Drug Approvals So Far in 2023
Last year, 37 novel drugs were approved by the FDA, this was a high number for such a category, and covered many fields including oncology, demonstrating how promising further research is, and how it is only continuing to build. To date, there are already 26 novel drug approvals (at time of writing), for half way through the year this is already a staggering number, showing just how successful the investments into innovations are in the industry. -
News FDA approves blood cancer therapeutic from AbbVie and Genmab
AbbVie and Genmab recently received approval from the US FDA for their joint venture, the blood cancer therapy epcoritamab, sold under the brand name EPKINLY.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










































































-comp273426.jpg)