PREFILLED SYRINGE (ENOXOPARIN) BIOSIMILAR
Product Description
Venus Pharma GmbH

-
DE
-
2019On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Venus Pharma GmbH

-
DE
-
2019On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Venus Pharma GmbH (5)
-
Product ANTIBIOTIC INJECTABLES Meropenem(FOR CRITICAL CARE)
CEFEPIME FOR INJECTION | CEFTRIAXONE | CEFTAZIDIME FOR INJECTION | CEFOPERAZONE + SULBACTAM FOR INJECTION | CEFUROXIME FOR INJECTION | CEFOTAXIME FOR INJECTION | CEFTAZIDIME AVIBACTAM FOR INJECTION | CEFTRIAXONE SULBACTAM FOR INJECTION | ELORES: CEFTRIAXONE, SULBACTAM & EDTA FOR INJECTION | AZTREONAM F... -
Product ONCOLOGY LIQUID INJECTABLES
CARBOPLATIN | CISPLATIN | CYTARABINE LIQUID | DOCETAXEL WITH SOLVENT | DOCETAXEL (CTD) SINGLE VIAL | DOXORUBICIN | EPIRUBICINE | ETOPOSIDE | IRINOTECAN | LEUCOVORIN CALCIUM | METHOTREXATE | OXALIPLATIN | PLERIXAFOR | PACLITAXEL | VINCRISTINE | 5-FLOUROURACIL | FULVESTRANT
-
Product ONCOLOGY LYOPHILIZED INJECTABLES
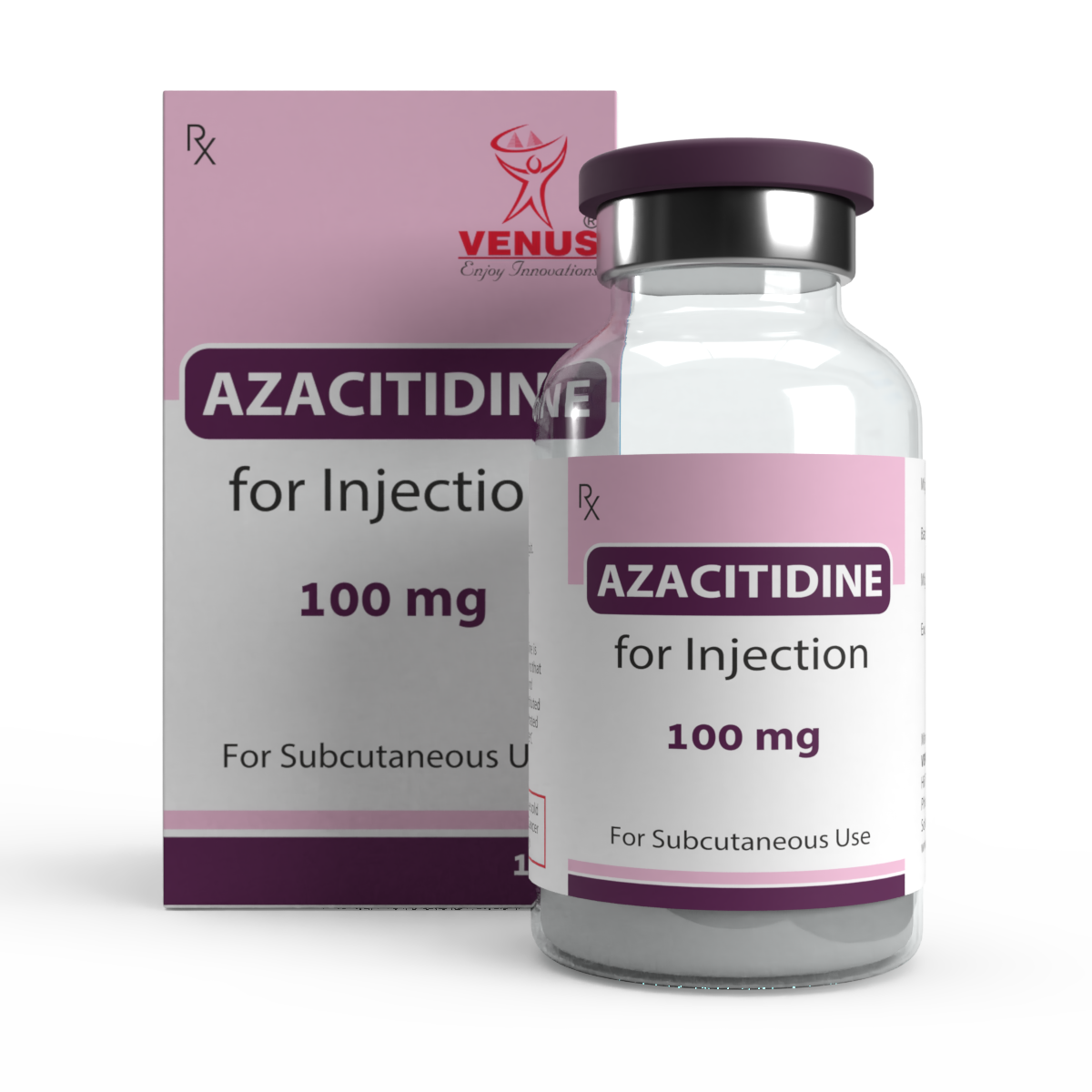
AZACITIDINE | BENDAMUSTINE | BLEOMYCIN | BORTEZOMIB | CYTARABINE | DACARBAZINE | EPIRUBICIN | GEMCITABINE | PEMETREXED | TOPOTECAN | ZOLENDRONIC ACID
-
Product Product List for Venus Remedies Limied-CPHI 2024
At VENUS REMEDIES LIMITED, we approach business partnerships with a strategic mindset, fostering collaboration that is not only successful in the short term but also sustainable and mutually beneficial in the long run. -
Product OTHER INJECTABLES
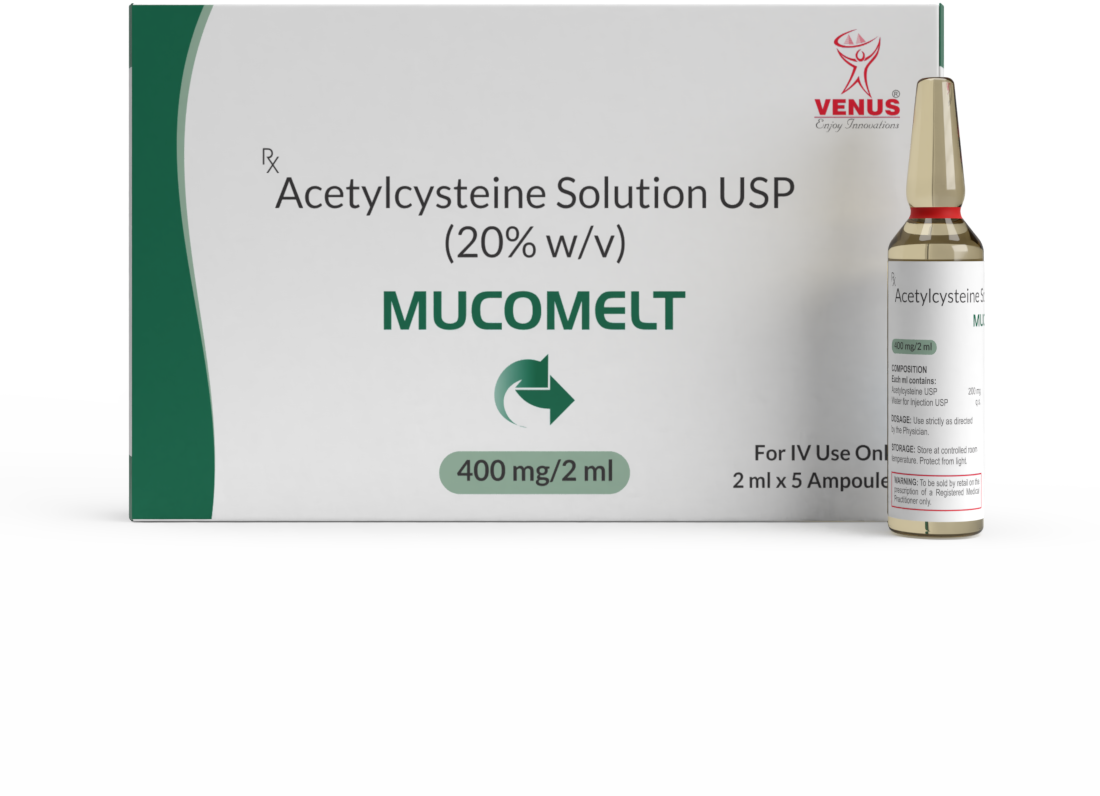
REMDESIVIR FOR INJECTION | ACECLOFENAC | CISATRACURIUM BESYLATE | CITICOLINE | DEXMETOMIDINE | L-ORNITHINE L-ASPARTATE | MIDAZOLAM INJ | N-ACETYLCYSTEINE | SUGAMMADEX| PARACETAMOL
Venus Pharma GmbH resources (8)
-
Brochure Stay tuned for more exciting updates!
Venus Remedies is making waves in the healthcare industry once again. We're bringing our latest groundbreaking innovations and global collaborations to the hear -
Brochure A Decade of Innovation: Venus Remedies and West Pharmaceutical Packaging @westat
A Decade of Innovation: Venus Remedies and West Pharmaceutical Packaging @westatwork Kriti Kotian, Marketing Specialist at West Pharmaceutical Packaging India -
Brochure Mr. Abednego Njalale - Ready to be at the forefront of healthcare innovation?
Join Venus Remedies and Venus Pharma GmbH at CPhI Milan 2024, from October 8th to 10th, in Hall 14, Meeting Rooms G52 & G54. -
Brochure Venus product list with Countries
Venus Product List with Countries -
Brochure Venus Remedies Global Presence -96+ countries
Venus Remedies Global Presence -96+ countries -
Brochure Venus Remedies Product List
At VENUS REMEDIES LIMITED, we approach business partnerships with a strategic mindset, fostering collaboration. -
Brochure VPG Product List for CPHI Milan
We approach business partnerships with a strategic mindset, fostering collaboration. -
Brochure Venus' Product List for CPHI 2024
Venus' Product List for CPHI 2024
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
.png)

















-file142565.png)