Pharmapack 2025: From the Floor in Paris

Pharmpack gears up for another week in Paris at the Porte De Versailles. The two-day show taking place on the 22–23 January, will cover contract packaging, device innovation, and sustainability among other topics.
Although the weather might be cold, there will be plenty of hot takes from the show in the following blog.
Sustainability is the first thing I'm talking about at Pharmapack this year, which feels appropriate as this is the first year we will be hosting a dedicated Sustainability Theatre in the conference side of things. We'll also see the return of the Sustainability Centre where our sustainability team will be based, and we bring the launch of the Sustainability Collective from CPHI to Pharmapack.
This year we are working with a number of Sustainability Partners to help make sustainability a memorable feature from the show. One of our partners is the environmental protection organisation Adelphe, who's work specifically focuses on paper and packaging materials. In an interview conducted prior to the show, Adelphe representative Vesna Reynal, Health Market Manager, highlighted what Adelphe consider the most pressing concerns in sustainability in pharmaceutical packaging today.
packaging materials. In an interview conducted prior to the show, Adelphe representative Vesna Reynal, Health Market Manager, highlighted what Adelphe consider the most pressing concerns in sustainability in pharmaceutical packaging today.
"Sustainability trends in packaging are growing, with a focus on recyclable mono-materials, bio-based alternatives (such as renewable materials) and lightweight design to reduce environmental impact as well as emphasis on reusable packaging."
See the full interview here.
 The content theatres are also covering the key aspects of pharmaceutical packaging across several tracks. The Device and Packaging Innovation track this year will be sponsored by Terumo, covering considerations such as patient adherence, the user journey, and a hollistic development of packaging and devices.
The content theatres are also covering the key aspects of pharmaceutical packaging across several tracks. The Device and Packaging Innovation track this year will be sponsored by Terumo, covering considerations such as patient adherence, the user journey, and a hollistic development of packaging and devices.
Thomas Isaac, Product Manager at Terumo says he is looking forward to "vivid discussions and an open exchange of ideas. It should be a thrilling track for all attendees."
See more of what Isaac has to say here.
Contract Packaging is a real topic of interest heading into 2025. This year we are featuring a full track on the topic, which will delive into some of the relationships with contract packaging organisations and CDMOs, and the elements of the supply chain involved in getting a product from clinic to market.
which will delive into some of the relationships with contract packaging organisations and CDMOs, and the elements of the supply chain involved in getting a product from clinic to market.
In an interview with Alexander Schäfer from Sharp Services, he states: "CPOs and CDMOs need to be more aligned as the industry needs to work together on some of our shared challenges, particularly around sustainability and sustainable materials. More supply chain collaboration will result in better outcomes for pharma when it comes to some of these challenges."
Read the full interview here.
Schäfer will be speaking on a panel: 'Panel Discussion: CPO and CDMO Strategic Visions' on Day 2 of the show at 11am in Conference Theatre G108. Make sure to grab your seat in the audience!
Day 1
We started the day in the Learning Lab, jumping straight into a very topical challenge that is currently facing the industry - GLP-1 production and scale-up.
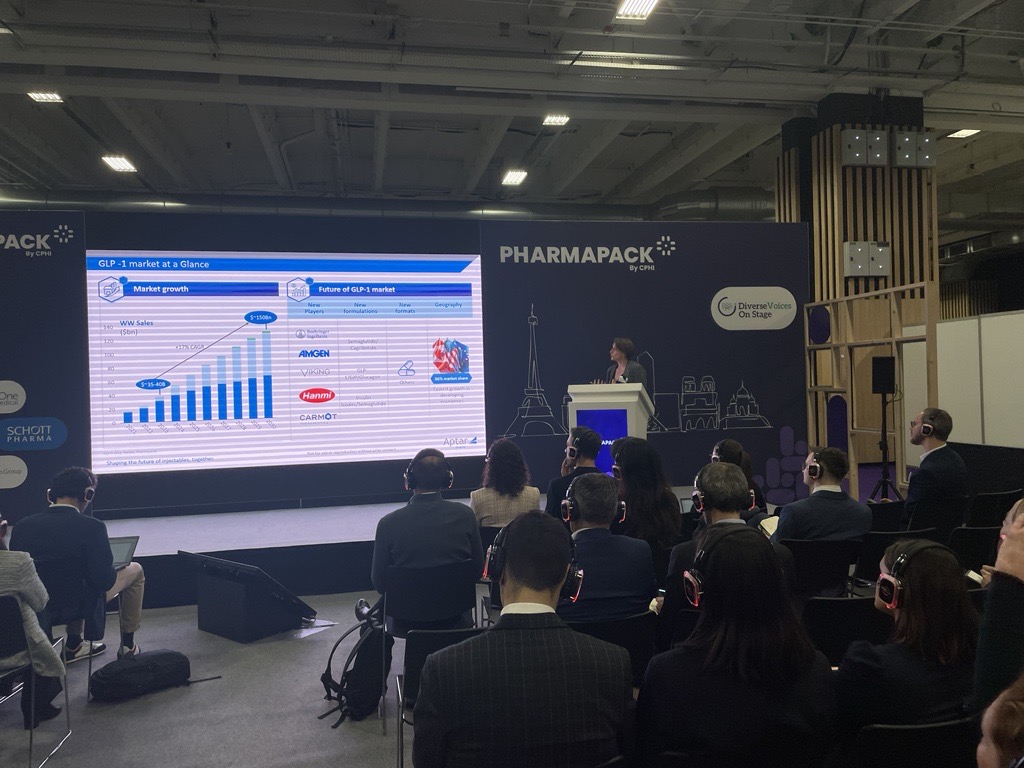 Audrey Chardonnet, from Aptar Pharma opens the session by giving some background to the use of GLP-1 agonists for the treatment of obesity and diabetes. She highlights the exorbitatnt growth the market has seen and the investment and interest from some of the biggest pharma companies. She states that this has meant an increased pressure on production, whilst also keeping in mind the quality and safety.
Audrey Chardonnet, from Aptar Pharma opens the session by giving some background to the use of GLP-1 agonists for the treatment of obesity and diabetes. She highlights the exorbitatnt growth the market has seen and the investment and interest from some of the biggest pharma companies. She states that this has meant an increased pressure on production, whilst also keeping in mind the quality and safety.
With the rising competition, developing sustainable options and increase patient adherence will be crucial to maintaining market popularity and growth.
"Multidose application using three different formats, autoinjectors, pen injectors, and vials, to maximise patient access and fit with market demand and trends.
Aptar pharma has been working to address the needs of the customer and support them when the market needs it the most."
In the Conference Theatre Content and Brand Director for Informa Tara Dougal opens the Device and Packaging Innovation track, introducing the keynote speaker, Lionel Jeannin.
What’s new in device and packaging innovation?.jpg)
Jeannin covers some of the challenges that need addressing for paper product and packaging, with size and use - therefore e-labelling eliminates the challenge of managing leaflets, which is a particular advantage when providing information for countries where there are multiple languages.
Jeannin covers some particular examples including the patient information e-leaflet provision in Singapore, including the surrounding regulation, and compares this to the situation in Australia, who have implemented a mandatory template to be followed when submitting a product.
Jeannin then moves on to other topics of high interest, speaking about Nitrosamines stating that since the awareness of nitrosamines impurities present in drug products, the industry has worked to analyse products and reduce the amount of contamination found through the manufacturing and packaging processes... What we need are packaging and device materials that our nitrosamine free. Some companies have already started to propose strategies to mitigate the risk of nitrosamine formation.
An interesting topic Jeannin highlights is Child Resistance, covering several guidelines in different countries, Australia, Saudi Arabia, and China where new mandates have been implemented to ensure increased safety around child resistant systems.
The keynote is following by a Lightning talk on Opthalimic drug delivery, giving a top line overview of the history of drug delivery devices in this area, and then looking to the present day innovations and beyond, a key take away being 'AI is the future of opthalmology' - food for thought!
Sprinting over to the brand new Sustainability Theatre, I caught the Keynote: Rethinking Sustainable Packaging - Turning costs into investments for transformative leadership, from Jayne Paramor of Anthesis.
.jpg) Rethink your whole system, of product placement, start from the outside in and get your distribution plan right, then work your way inwards to the areas internally that are causing the most problems.
Rethink your whole system, of product placement, start from the outside in and get your distribution plan right, then work your way inwards to the areas internally that are causing the most problems.
Rethink the product - can you reformulate for example a gell capsule to a hard capsule that then can be packaged in a recyclable bottle rather than a blister packet?
Educate stakeholders - everyone needs to understand why they need to be on board with sustainability so that they can progress along the journey with a company.
Next in the Sustainability Theatre, Fatima Bennai-Sanfourche from Bayer AG gives a talk on 'Global Regulatory Strategy: Integrating Sustainability Compliance'. Bennai-Sanfourche covers several initiatives that can be used to increase compliance and reduce waste and emmissions across several aspects.
Strategies that she sets out include developing robust policies (including anticipating guidelines and training stakeholders on this), continuous monitoring, and developing a framework for compliance.
The morning has flown by and we're back in the Conference Theatre being entertained by AstraZeneca and Team Consulting in the form of Andrew Chapman and Tom Etheridge respectively, with their Keynote on 'Reusability Simplified: The Journey to Develop a Sustainable, Low-Cost Reusable Autoinjector'. .jpg)
The pair provided a case study on the development of an innovative autoinjector 'The AstraZeneca Reuseable Autoinjector', taking us through design, build, and testing phases.
Chapman demonstrated onstage the autoinjector, showing how simple the 4-step use of the device is.
Etheridge detailed some of the key challenges that the team encountered on their development journey. They found that several users would mis-orient the cassette, so they created 4-way cassette symmetry. They have developed an easy-grip cap, and poke yoke features to enhance ease of use.
A question from the audience asked whether the team had considered the longevity of the spring system in their development? Chapman stated that they had considered it in development but hadn't had the chance to complete full life-time testing, but felt confident that the device would maintain integrity for several years and continued use.
The final session before lunch was a panel discussion, 'Device and Packaging Innovation: Toward Customer-Centric Commercialisation', with Robert Greene, a Patient Advocate Consultant, Serkan Oray, UCB, and Tom Oakley from Sanner Group.
.jpg) Greene makes a good point about how people often question why speak to the patients, wondering what questions they could answer, however he says if you speak to the patients first, and then look at what that informs.
Greene makes a good point about how people often question why speak to the patients, wondering what questions they could answer, however he says if you speak to the patients first, and then look at what that informs.
Oakley asks what are the biggest areas of improvement for customers?
One trend that Oray is seeing at the minute is earlier involvement in clinical studies, which is differenitiating and important for using devices earlier in the life cycle, and producing more volume in the future.
Oray states that the requirements across geographies diverges quite a bit, therefore it requires more to meet the specific needs of the different areas.
Oakley brings the conversation to representation, asking Greene if there is more to be done to address issues around underrepresentation of certain groups in clinical trials.
Greene says it comes down to understanding who the medicines are being used for, once you understand who will be using the medicines then the best way to be informed about their specific needs is to ask those patients and give them a chance to inform design and development.
Moving into the afternoon we start the Large Volume Drug Delivery Track. The Keynote Address 'Towards New Devices or New Formulations?' is given by the brilliant Asmita Khanolkar from SMC Pharma Services.
This is followed by a lightning talk from Mayur Patel from PA Consulting: 'Regulatory and Clinical Trial Approaches for On-Body Injectors'.
Patient compliance issues including attachment of the device, achieving the correct dosage, these are all challenges that need to be addressed and mitigated. .jpg)
Another Lightning Talk follows: 'Injection Tolerability - Understanding the Influence of Device Design on Injection Pain' from Andrew Fiorini of the Cambridge Design Partnership.
Fiorini discusses the nuances of the topic, giving overviews of the focus of clinical trials that have tested this concept, and challenges with questions around timeline of injections, does size matter and patient adherence.
As much as you learn in the conference theatres, there's always so much to discover by walking the floor of the show, even if it's not what you expect, such as earlier when I quite literally bumped into a friend from EMA Pharma:
.jpg) Catch them at booth G65 to find out more about the solution behind the costume!
Catch them at booth G65 to find out more about the solution behind the costume!
Moving on in the Large Volume Drug Delivery Track we hear from Pharmapack favourite, Aurelio Arias, Director at IQVIA on: 'Keynote: Transforming Healthcare by Embracing Subcutaneous Innovation'.
Arias looks at the benefits of developing subcutaneous formulations, looking at several pharma companies' strategies, whether it is in speeding up treatment, biosimilar defence, or as a novel offering. The benefits of such strategies turned out to be numerous, including the reduction in administration time, a great benefit for patients, a huge cost saving for the patient, and the provision of an additional treatment option.
Closing the day in the Sustainability Theatre we had a final.jpg) panel with 'Partnering for Success: Proactive Supplier Collaboration Across the Pharma Ecosystem for Combination Product Success' with Julien Tremblin, Alexander Schäfer, Barbara Huist, Nic Hunt, Samantha Smith, and Sophie Ruddick.
panel with 'Partnering for Success: Proactive Supplier Collaboration Across the Pharma Ecosystem for Combination Product Success' with Julien Tremblin, Alexander Schäfer, Barbara Huist, Nic Hunt, Samantha Smith, and Sophie Ruddick.
Partnerships are almost becoming synonymous with sustainability in the industry, with companies recognising that impactful changes across the whole ecosytem can only really happen if we work together.
.jpg) As per tradition, we finish the first day of the show with the Pharmapack Awards ceremony, where we take the time to recognise innovators in the industry that have demonstrated steps to push the industry forward.
As per tradition, we finish the first day of the show with the Pharmapack Awards ceremony, where we take the time to recognise innovators in the industry that have demonstrated steps to push the industry forward.
The evening is a great time to finally have a few drinks, listen to live music, and decompress from a very busy and exciting day!
See all the winners and what each of them had to say on receiving their awards here.
Congratulations to all of the winners from this year's show!
.jpg) Now it's been a long day, we've walked for what feels like miles, time to take a leaf out of Director Tara Dougal's book and kick back and relax before we do it all again tomorrow!
Now it's been a long day, we've walked for what feels like miles, time to take a leaf out of Director Tara Dougal's book and kick back and relax before we do it all again tomorrow!
Day 2
The second day of the conference was an early start for the entusiastic among us. A 6am wake up for a morning run to the Eiffel Tower. The group set off nice and early, watching the city wake up while learning a little bit about the history of Paris from our tour guide. Making it to the iconic landmark before the sun was even up!
.jpg)
.jpg)
Back on the show floor after the morning's outing and after secretly wishing I could spend the whole day in one of the massage chairs I was pleasantly distracted by breakfast with the Sustainability Collective at the Sustainability Centre. This was a fantastic opportunity to catch up with sustainability friends as well as meet some new people in the field.
After breakfast I spent some time walking the halls and speaking with exhibitors and speakers until it was time to take the stage myself to moderate a panel in the Sustainability Theatre: 'Panel Discussion: Sustainability of the Future: Innovating Together'.
The panellists consisted of Kami Krista, Janet Smith, Mary Poddar, Nicolas Longhitano, and Sandra Matamoros, ensuring a wide range of expertise, covering the enture supply chain.
The panel covered various aspects of sustainability within the supply chain, where the biggest areas of concern and biggest areas of potential were, briefly touching on how the current political climate might affect green progress in the future. A point that kept cropping up was collaboration, the importance of this for enabling practical strategies, around reporting, renewable energy, and circularity, and for really being able to make a difference industry-wide in the time we have left to do so. .jpg)
Related News
-
News Women in Pharma: Connecting accessible pharma packaging to patients – a Pharmapack Special
Throughout our Women in Pharma series, we aim to highlight how CPHI events encourage discussions around diversity, equity, and inclusion initiatives in the pharmaceutical industry. -
News Pharma contract packaging, a future focusing on collaboration
Contract packaging in pharmaceuticals is more than just what kind of blister packaging you want to go for, there are so many key elements that depend on a niche of needs and regulations. This article focuses on what partnerships in the field are focusi... -
News CPHI Podcast Series: Packaging expert perspectives at Pharmapack 2025
This month's podcast episode sounds a little different, covering the latest event in Paris – Pharmapack 2025. Digital Editor Lucy Chard speaks to several experts direct from the floor of the show, bringing you right in on the action.&nbs... -
News The 2025 Pharmapack Awards: recognising innovation and patient centricity
This year in Paris the Pharmapack Awards recognised the achievements across categories in the industry, aswell as including some new categories to highlight exceptional work and people. -
News Visibility, Integration, and Opportunity with CPOs: A Pharmapack Interview
At Pharmapack 2025 in Paris the informative content tracks this year will feature a Contract Packaging track. A critically important topic for those working in the field, which speaker Alexander Schäfer from Sharp Services discusses in the fo... -
News Pharmapack Sustainability Partner Interview: Sustainable blister packaging
At Pharmapack we are increasingly aiming to bring sustainability into the event, into the conversations people have, into the content we present and into the running of the show itself. This year we are working with a number of Sustainability Partners ...
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance