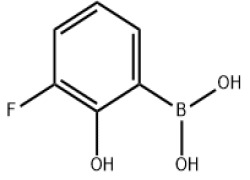
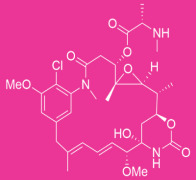
Oncology
Product Description
QUOTIENT SCIENCES LIMITED

-
GB
-
2017On CPHI since
-
500 - 999Employees
Company types
Categories
Specifications
QUOTIENT SCIENCES LIMITED

-
GB
-
2017On CPHI since
-
500 - 999Employees
Company types
More Products from QUOTIENT SCIENCES LIMITED (16)
-
Product Drug Development Consulting
Our Drug Development Consultants work with you to design and implement successful drug development programs. With industry leading scientific expertise across a range of technical disciplines, we help customers across all stages of development, from candidate selection to commercial launch.
Our cons... -
Product Global Clinical Trial Supply
Accelerate your timeline, with flexible, on-demand delivery. We’ll design and develop your packaging, labelling and distribution strategy for Phase I, Phase II and Phase III clinical studies, so you’ll no longer have to worry about the challenge of getting your drug product to multiple sites in time fo... -
Product Pediatric Development
Pediatric formulation and product development
The development of acceptable, palatable pediatric formulations is a key feature within the industry today, driven by patient needs and regulatory requirements. Quotient Sciences has extensive knowledge and capabilities that enable us to provide you... -
Product Orphan Drug Development
Accelerating orphan drug development from candidate selection to commercial manufacture and supply.
With over 300 million people worldwide living with identified rare diseases, the development of new treatments to address these unmet clinical needs is an area of great interest in the pha... -
Product Inhaled Drug Development
Quotient has a 30 year track record in the development and manufacturing of inhaled drug products. We have experience across a range of drug delivery platforms including dry powder inhalers (DPIs) and solutions/suspensions for inhalation. With integrated capabilities and vast knowledge of nasal and pu... -
Product Translational Pharmaceutics - Integrated Programs
Translational Pharmaceutics® accelerates drug development by integrating formulation development, real-time manufacturing and clinical testing. The platform is unique to Quotient Sciences and has been used over the last decade by global pharmaceutical and biotech companies across over 400 drug programs. qq... -
Product Candidate Selection - Integrated Programs
Selecting the right molecules for development - Quickly identify the best drug candidates. Knowing what it takes to develop a successful drug, we help clients select the best molecules for development. Our unique integration of scientific capabilities enables us to provide a complete assessment o... -
Product Early Stage Development - Integrated Programs
Accelerating molecules through to proof-of-concept - Simplify early development and accelerate to Proof-of Concept.Proof-of-concept (POC) is a key milestone in the development of a new drug candidate. We understand the transition to POC needs to be fast and cost-effective.With fully integrated capabil... -
Product Late Stage Development - Integrated Programs
.Accelerating products through to commercial manufacture - Speed the journey from proof-of-concept to commercial launch. The journey from proof-of-concept to commercial launch can be complex. Speed is critical but it shouldn’t come at the expense of product quality. Creating patient-centric formulatio... -
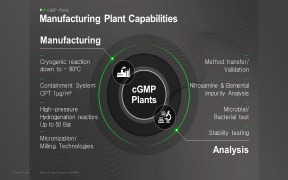
Product Drug Substance
Our streamlined approach to developing drug substance has been shown to reduce manufacturing costs by 50% from preclinical to Phase I. To best support our customer’s needs, we have made significant investments in technologies and equipment to meet the increasing demand for complex small-scale dr... -
Product Formulation Development
Quotient Sciences has almost 30 years of experience developing a breadth of pharmaceutical formulations across a range of indications. Our innovative approach to formulation development integrates drug product development with clinical evaluation, combined with our experience with over 1,000 molecules at a... -
Product Clinical Trial Manufacturing
Understanding that early phase clinical testing is a pivotal milestone in the development of your drug product, Quotient offers a clinical trial manufacturing, testing and certification service designed to meet your individual requirements. Our innovative method of building integrated GMP and GCP programs ...
QUOTIENT SCIENCES LIMITED resources (25)
-
News CPHI Milan 2024: Excerpts from the Exhibitors
After another successful year of bringing the pharmaceutical community together at CPHI Milan in October, hear direct from the exhibitors on why the event is so important for them and the industry as a whole. -
Datasheet Formulation Development at Quotient
Quotient Sciences has almost 30 years of experience developing a breadth of pharmaceutical formulations across a range of indications. Our innovative approach to formulation development integrates drug product development with clinical evaluation, combined with our experience with over 1,000 molecules at all stages of drug development. -
News Quotient Sciences acquires UK-based CDMO Arcinova
With over 40 years of experience and 160 employees, Arcinova provides drug substance, drug product and bioanalysis services to over 200 pharma and biotech customers worldwide -
Datasheet Clinical Pharmacology at Quotient
When you are looking for a partner who is dedicated to Phase I trials and early development,rely on Quotient Sciences.
We accelerate your molecule from first-in-human to proof-of-concept,helping you make critical decisions earlier.
Whatever clinical pharmacology study you require, you can expect a fully integrated program from study design to data reporting. -
News Quotient Sciences Completes Million Dollar Pharmacy Expansion at Miami Clinic
Miami, FL/June 22, 2020 - Quotient Sciences, a global pharmaceutical development, clinical and commercial manufacturing organization, announced today the completion of a $1 million dollar pharmacy and laboratory expansion at their Miami, FL clinical pharmacology facility.
The expanded pharmacy capabilities offer customers a quick and cost-effective way to start clinical testing, without the need for extensive CMC investment or API consumption at this early stage of development. -
Datasheet Global Formulation & Manufacturing Capabilities
With over 30 years’ experience, Quotient Sciences provides full service support for small molecule non-sterile programs, from dosage formulation development through to GMP clinical trial manufacturing and commercial drug product supply. -
News Quotient Sciences and CytoAgents Accelerate Potential Treatment for COVID-19 Cytokine Storm
Collaboration Expedites Human Clinical Trials for COVID-19 Drug Candidate. A collaboration to accelerate the development of a lead COVID-19 drug candidate into human clinical trials was announced today [April 28, 2020] by Quotient Sciences, a leading provider of innovative drug development and manufacturing solutions, and CytoAgents, Inc. a privately help biotechnology company focused on the development of pharmaceutical products for treatment of infectious diseases. -
Datasheet Clinical Trial Manufacturing at Quotient
Understanding that early phase clinical testing is a pivotal milestone in the development of your drug product, Quotient offers a clinical trial manufacturing, testing and certification service designed to meet your individual requirements.
Our innovative method of building integrated GMP and GCP programs provides you with a streamlined, flexible approach to drug product supply that reflects your clinical study design and timeline.
We understand the time and cost pressures you face during early phase evaluation and work with you to ensure a rapid, seamless path from development to clinical trial supply. -
News ANA Therapeutics and Quotient Sciences Announce Partnership to Manufacture Niclosamide Drug Candidate as a Potential Treatment for COVID-19 Collaboration
ANA Therapeutics, a Silicon Valley-based biotech startup, and Quotient Sciences, a leading provider of innovative drug development and manufacturing solutions, today announced a partnership to support the manufacturing of ANA Therapeutics’ drug candidate, ANA001 (niclosamide capsules), which they are developing as a potential treatment for COVID-19. -
Datasheet Commercial Manufacturing at Quotient
Pharmaceutical commercial manufacturing - Quotient Sciences is a global player in commercial drug product manufacture of small molecule products for niche therapies including oncology and orphan drugs.
Our commercial manufacturing facility located in Philadelphia is designed to handle your high-potency compounds. -
Datasheet Pediatric Development at Quotient
Pediatric formulation and product development
The development of acceptable, palatable pediatric formulations is a key feature within the industry today, driven by patient needs and regulatory requirements.
Quotient Sciences has extensive knowledge and capabilities that enable us to provide you with a unique integrated pediatric development solution.
-
Datasheet Taste Masking at Quotient
Many drug substances are extremely bitter or have other aversive attributes,which can make developing palatable drug products extremely challenging.This is a common problem seen in medicines spanning all therapeutic areas,from antibiotics and painkillers to antihistamines and decongestants. The careful design and development of formulated oral drug products is key to ensuring patient acceptability and compliance for achieving the desired clinical outcomes. -
Datasheet 505(b)(2) Product Development at Quotient
Are you developing a new dosage form for an existing drug?
Do you need to explore a different route of administration?
Quotient has significant experience in 505(b)(2)product development and can support you in efficiently turning your innovative ideas into successful products.Over the past several years, the FDA’s 505(b)(2)regulatory pathway has enabled the approval of a variety of differentiated dosage forms for existing molecules -
Datasheet Translational Pharmaceutics - a Quotient Innovation
Translational Pharmaceutics® accelerates drug development by integrating formulation development, real-time manufacturing and clinical testing. The platform is unique to Quotient Sciences and has been used over the last decade by global pharmaceutical and biotech companies across over 400 drug programs. -
Datasheet PBPK Modelling and Simulation at Quotient
Quotient is a leading expert in the application of physiologically based pharmacokinetic (PBPK) modelling and simulation (M&S) science to drug development.
Using GastroPlus™ we advise our clients on the potential in vivo performance of drugs and formulations to inform product and clinical development strategies.
Whether working in a consultancy relationship or as part of an integrated program of work, our PBPK expertise is underpinned by our unique and extensive experience in pharmaceutics, biopharmaceutics, DMPK and clinical research. -
Datasheet Pre-formulation & Material Sciences at Quotient
Providing expertise to support pre-formulation development
Our formulation development and material sciences experts have over 30 years’ experience in pre-formulation and solid state characterization. -
Datasheet Compounding and GMP Manufacturing for Product Supply at Quotient
Quotient Sciences provides integrated pharmacy compounding, formulation, GMP manufacturing and clinical testing solutions to help clients achieve their proof-of-concept (POC) milestone quickly, saving them precious development time and money.
Begin your First-in-Human (FIH) Phase I testing with a fit-for-purpose, simple pharmacy preparation, and then seamlessly transition to a scalable, solid oral GMP drug product for Phase II trials, all within one organization. -
Datasheet Inhaled Product Development at Quotient
With more than 30 years’ experience, Quotient is a leader in the development, manufacture and assessment of inhalation products, helping clients to accelerate molecules from first-in-human through to proof-of-concept.
Our integrated capabilities and vast knowledge encompasses pre-formulation sciences, formulation development, device evaluation, clinical trial manufacturing and the clinical assessment of a variety of inhaled formats for nasal and pulmonary delivery. -
Datasheet High Potency Handling at Quotient
High Potency Drug Development & Manufacturing
Highly potent active pharmaceutical ingredients (HPAPIs) are becoming increasingly common in drug development pipelines, especially in the oncology sector, as researchers search for therapies with greater selectivity and pharmacological activity. However, HPAPIs present additional CMC challenges due to the containment requirements to protect both the operators and manufacturing facilities. Their production processes may also require greater precision and control due to the very small quantities of drug present in the final dosage form. -
Datasheet Human ADME Studies at Quotient
Whatever your requirements, Quotient Sciences can support you in the generation of clinical metabolism data for your drug development program and for NDA, MAA and global regulatory filings. -
Datasheet First-in-human to Proof-of-Concept at Quotient
Quotient Sciences’ Translational Pharmaceutics® approach enables First-in-Human studies by re-engineering the transition of your drug molecule into clinical development and shortening the timeline to Proof-of-Concept.
How? Quotient’s Translational Pharmaceutics® platform integrates formulation development, real-time adaptive GMP manufacturing and clinical testing so that drug product manufacture occurs immediately prior to dosing.Each manufacture of drug product is therefore conducted in response to emerging clinical data. This approach delivers significant benefits to the development team. -
Datasheet Spray Drying at Quotient
Overcoming poor drug solubility and bioavailability challenges
Drug solubility has a significant impact on bioavailability. 70% of new chemical entities (NCEs) suffer from low aqueous solubility that can result in failure during clinical testing due to poor bioavailability. Selecting an appropriate drug formulation is imperative to the success of the program. -
Datasheet Project Management at Quotient
Project Management at Quotient – A True Partnership
Quotient is an innovative drug development and manufacturing partner supporting clients from candidate selection to commercial launch. With expertise in end-to-end Project Management and integrated project teams, we make drug development easier for our customers and dramatically reduce the time and cost of getting new medicines to patients. -
Video John McDermott, Quotient Sciences, discusses the unique benefits of Translational Pharmaceutics
John McDermott, Executive Director, Drug Development Solutions - Quotient Sciences discusses Translational Pharmaceutics at CPHI Worldwide, and how a recent Tufts study has proven the significant savings and financial benefits for drug developers who use Translational Pharmaceutics. -
Datasheet Formulation Strategies for Poorly Soluble Molecules at Quotient
As the number of poorly soluble compounds continues to increase in the industry development pipeline, conventional formulation strategies may not be sufficient to achieve acceptable levels of solubility in the gastrointestinal tract and hence absorption into the systemic circulation. Scientists will need to utilize advanced formulation technologies to maximize oral bioavailability.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance









































.jpg)


































%20(1)-comp286402.jpg)