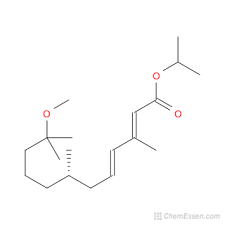
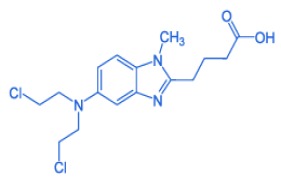
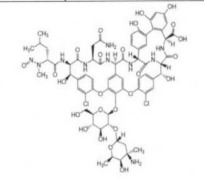
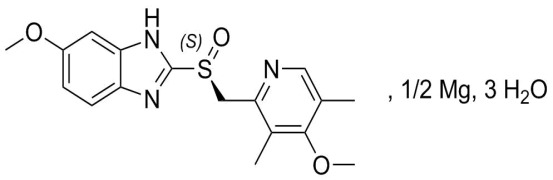
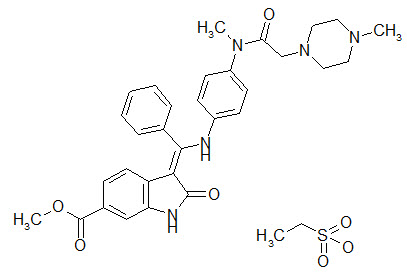
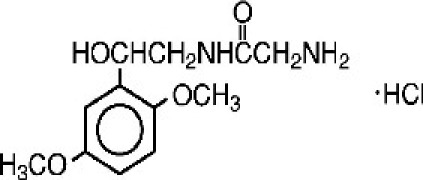
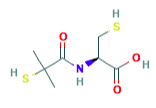
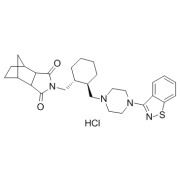
Nintedanib Esylate
Product Description
OMGENE LIFE SCIENCES PRIVATE LIMITED

-
IN
-
2020On CPHI since
-
50 - 99Employees
Company types
Categories
Specifications
OMGENE LIFE SCIENCES PRIVATE LIMITED

-
IN
-
2020On CPHI since
-
50 - 99Employees
Company types
More Products from OMGENE LIFE SCIENCES PRIVATE LIMITED (44)
-
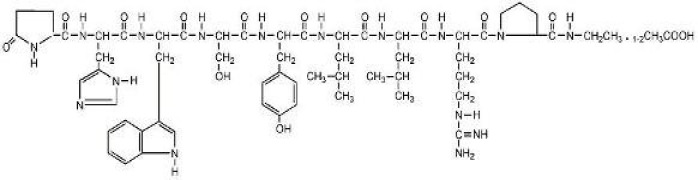
Product Leuprolide Acetate
Indicated in the palliative treatment of advanced prostatic cancer. -
Product Linaclotide
Indicated in adults for the treatment of irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC) -
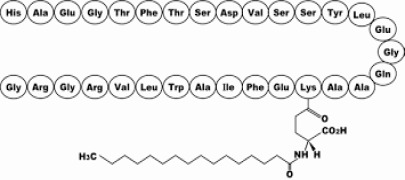
Product Liraglutide
Indicated as an adjunct to diet and exercise to improve glycemic control in patients 10 years and older with type 2 diabetes mellitus, to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fat... -
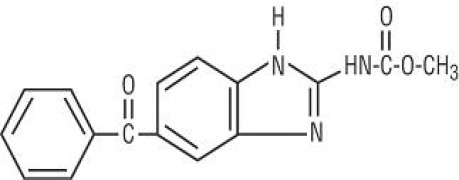
Product Mebendazole (Micronized)
Indicated for the treatment of patients two years of age and older with gastrointestinal infections caused by Ancylostoma duodenale (hookworm), Ascaris lumbricoides (roundworm), Enterobius vermicularis (pinworm), Necator americanus (hookworm), and Trichuris trichiura (whipworm). -
Product Midodrine Hydrochloride
Indicated for the treatment of symptomatic orthostatic hypotension (OH). -
Product Midostaurin
Indicated, in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by a FDA approved test -
Product Nitisinone
Indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine. -
Product Obeticholic acid
Indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults with an inadequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA. -
Product Octreotide Acetate
Indicated for treatment of Acromegaly, Carcinoid Tumors, VIP-secreting tumors. -
Product Oxytocin Acetate
For the temporary relief of symptoms of food cravings, including: constant appetite, increased or insatiable hunger. The peripheral actions of oxytocin mainly reflect secretion from the pituitary gland.
Purity not les sthan 99% Complete impurity characterization available
-
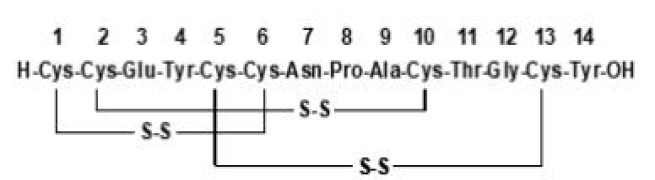
Product Pentetreotide
Indium In-111 pentetreotide is an agent for the scintigraphic localization of primary and metastatic neuroendocrine tumors bearing somatostatin receptors. -
Product Plecanatide
Indicated in adults for the treatment of: chronic idiopathic constipation (CIC). irritable bowel syndrome with constipation (IBS-C)
OMGENE LIFE SCIENCES PRIVATE LIMITED resources (3)
-
Brochure Brochure of Company
Brochure of the company for referring to Company's work profile, strengths, capabilities and facility information. -
Brochure Product List
To view the list of products we are working on, do contact us for any specific enquiry or clarity. Looking forward for further communication and collaboration. -
Brochure Omgene API
For referring to Company's work profile, strengths, capabilities, facilities etc
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




































































%20(1)%20(1)-comp246058.png)