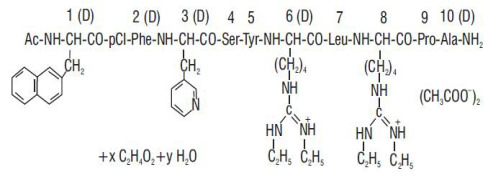
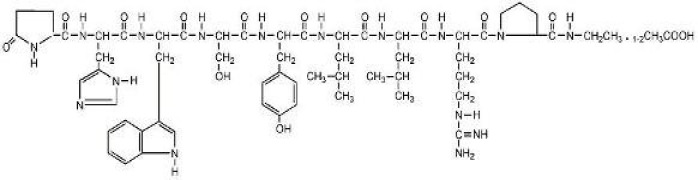
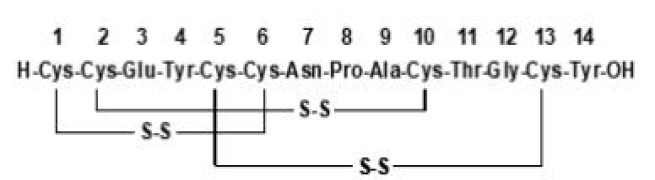
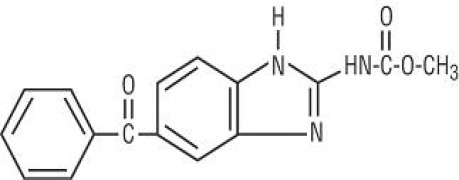
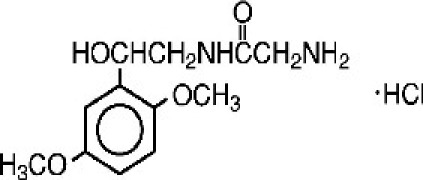
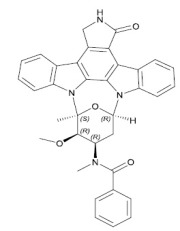
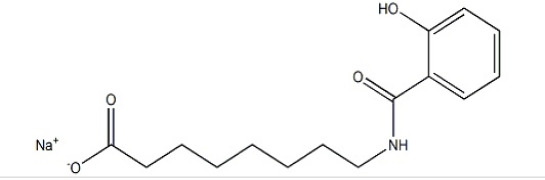
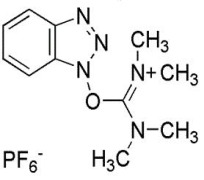
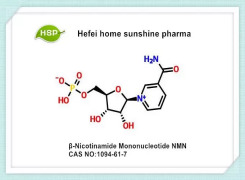
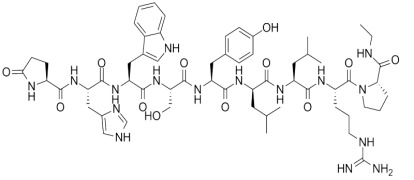
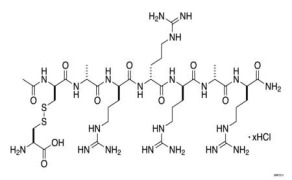
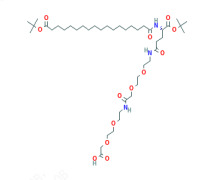
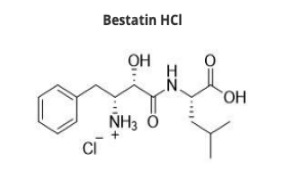
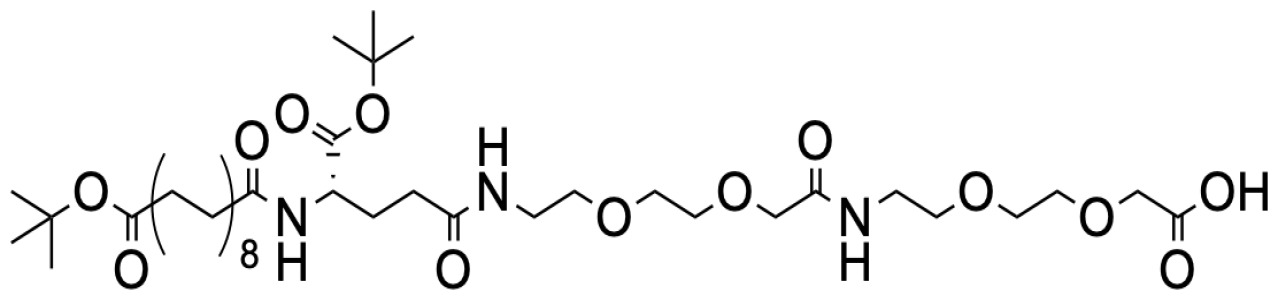
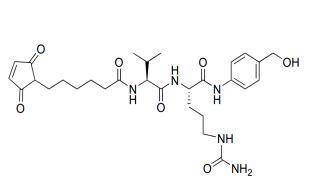
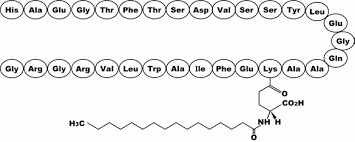
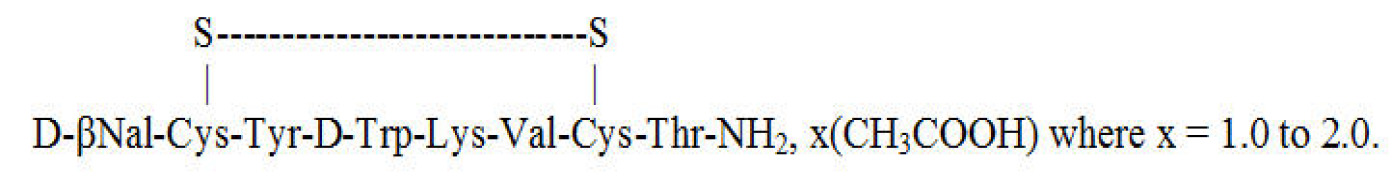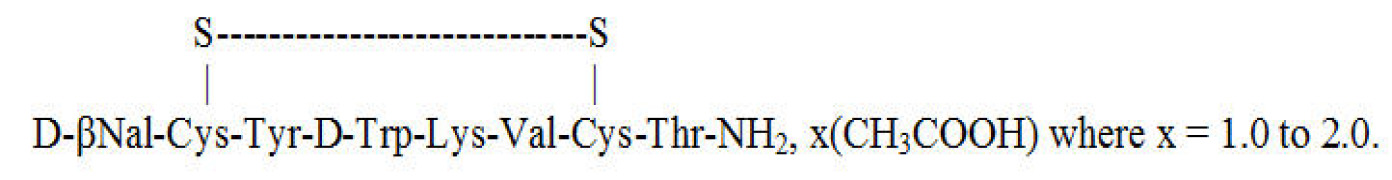Liraglutide
Product Description
OMGENE LIFE SCIENCES PRIVATE LIMITED

-
IN
-
2020On CPHI since
-
50 - 99Employees
Company types
Categories
Specifications
OMGENE LIFE SCIENCES PRIVATE LIMITED

-
IN
-
2020On CPHI since
-
50 - 99Employees
Company types
More Products from OMGENE LIFE SCIENCES PRIVATE LIMITED (44)
-
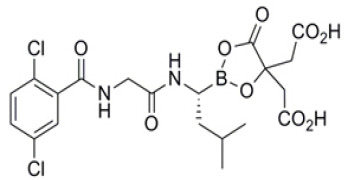
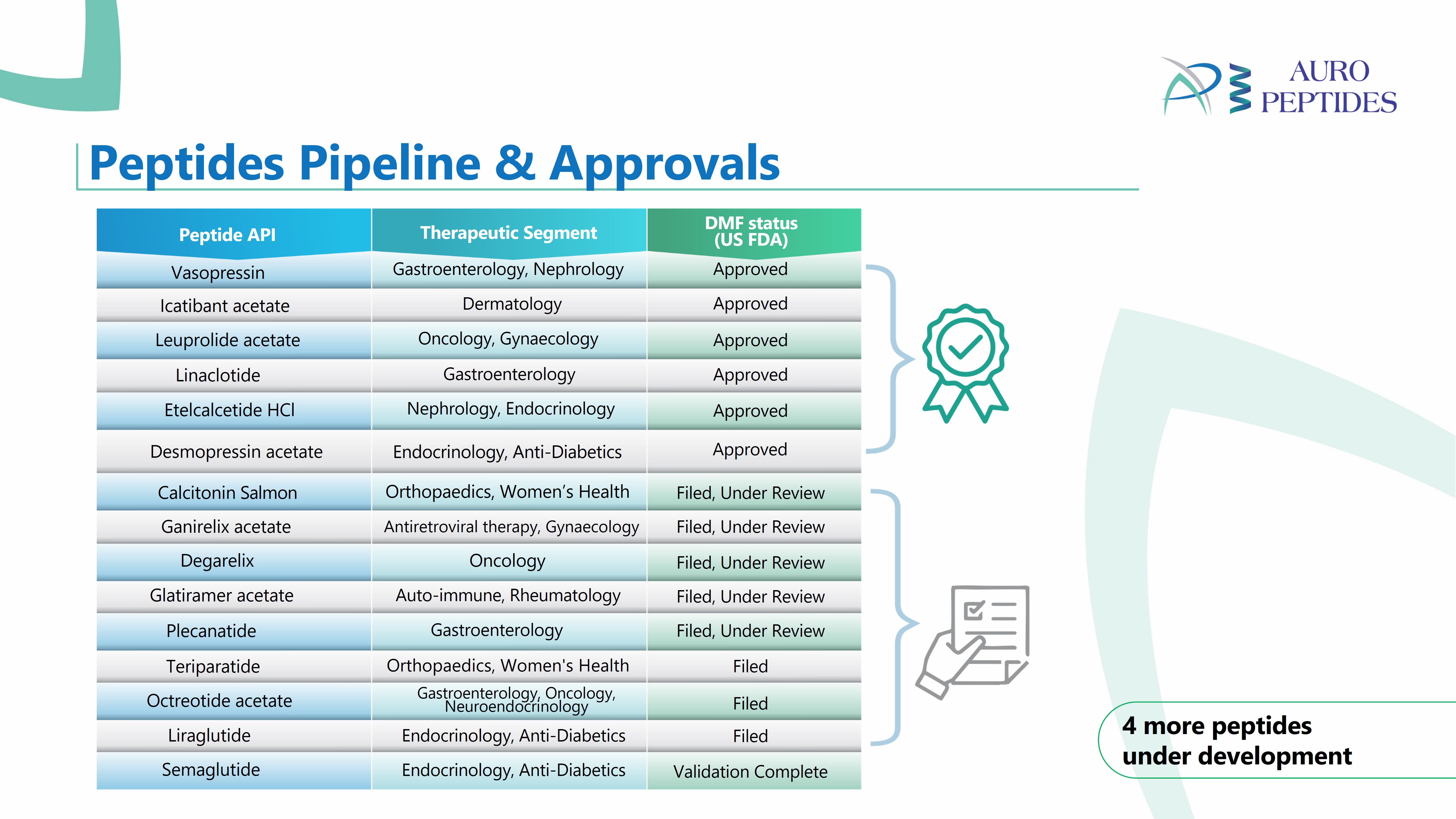
Product Ganirelix Acetate
Indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation. Close -
Product Ixazomib Citrate
Indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy. -
Product Lanreotide Acetate
Indicated for the long-term treatment of acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option. -
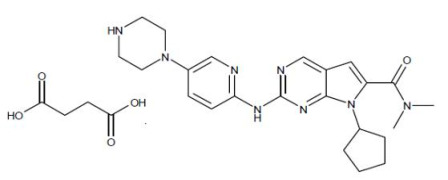
Product Ribociclib Succinate
Indicated in combination with an aromatase inhibitor for the treatment of pre/perimenopausal or postmenopausal women, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, as initial endocrine-based therapy; or fulvestrant ... -
Product Leuprolide Acetate
Indicated in the palliative treatment of advanced prostatic cancer. -
Product Linaclotide
Indicated in adults for the treatment of irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC) -
Product Mebendazole (Micronized)
Indicated for the treatment of patients two years of age and older with gastrointestinal infections caused by Ancylostoma duodenale (hookworm), Ascaris lumbricoides (roundworm), Enterobius vermicularis (pinworm), Necator americanus (hookworm), and Trichuris trichiura (whipworm). -
Product Midodrine Hydrochloride
Indicated for the treatment of symptomatic orthostatic hypotension (OH). -
Product Midostaurin
Indicated, in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by a FDA approved test -
Product Nintedanib Esylate
Indicated for treatment of Idiopathic Pulmonary Fibrosis, Chronic Fibrosing Interstitial Lung Diseases with a Progressive Phenotype, Systemic Sclerosis-Associated Interstitial Lung Disease -
Product Nitisinone
Indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine. -
Product Obeticholic acid
Indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults with an inadequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA.
OMGENE LIFE SCIENCES PRIVATE LIMITED resources (3)
-
Brochure Brochure of Company
Brochure of the company for referring to Company's work profile, strengths, capabilities and facility information. -
Brochure Product List
To view the list of products we are working on, do contact us for any specific enquiry or clarity. Looking forward for further communication and collaboration. -
Brochure Omgene API
For referring to Company's work profile, strengths, capabilities, facilities etc
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance