Nanosol
Product Description
Ascendia Pharmaceuticals

-
US
-
2017On CPHI since
-
2Certificates
-
50 - 99Employees
Company types
Categories
Ascendia Pharmaceuticals

-
US
-
2017On CPHI since
-
2Certificates
-
50 - 99Employees
Company types
More Products from Ascendia Pharmaceuticals (19)
-
Product Lyophilization
Ascendia pharmaceuticals offers lyophilization. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
Product Micro-fluidizer
Ascendia pharmaceuticals offers micro-fluidizer. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
Product Nano-particle bead-milling
Ascendia pharmaceuticals offers nano-particle bead-milling. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
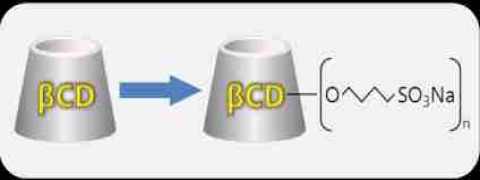
Product Nanosol
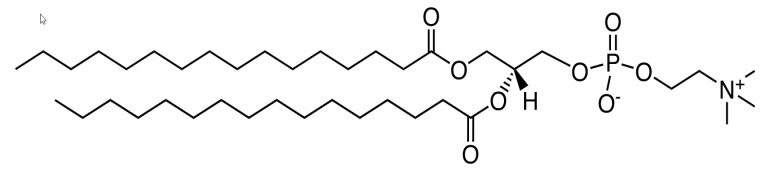
Ascendia pharmaceuticals offers nanosol. Features: it is a technology for the production of nano-sized drug particles. Formulation of a drug substance in nano-particle form significantly increases the surface area available for dissolution. Contact us for more information. -
Product Nanosol
NanoSol is a technology for the production of nano-sized drug particles. Formulation of a drug substance in nano-particle form significantly increases the surface area available for dissolution. A ten-to-twenty-fold increase in surface area can result from reducing particle size from a micronized dru... -
Product Nanosol
NanoSol is a technology for the production of nano-sized drug particles. Formulation of a drug substance in nano-particle form significantly increases the surface area available for dissolution. A ten-to-twenty-fold increase in surface area can result from reducing particle size from a micronized dru... -
Product Nmr and xrpd
Ascendia pharmaceuticals offers nmr and xrpd. Features: it is an analytical instrument for testing pharmaceutical formulations. Contact us for more information. -
Product Particle size distribution analysis
Ascendia pharmaceuticals offers particle size distribution analysis. Features: it is an analytical instrument for testing pharmaceutical formulations. Contact us for more information. -
Product Roller compaction
Ascendia pharmaceuticals offers roller compaction. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
Product Solvent evaporation
Ascendia pharmaceuticals offers solvent evaporation. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
Product Spray dryer
Ascendia pharmaceuticals offers spray dryer. Features: it is an equipment for producing pharmaceutical formulations. Contact us for more information. -
Product Stability chambers
Ascendia pharmaceuticals offers stability chambers. Features: it is an analytical instrument for testing pharmaceutical formulations. Contact us for more information.
Ascendia Pharmaceuticals resources (4)
-
News Pandemic and transformative technologies fuel growth in pharma outsourcing sector: CPHI North America panel
Transformative technologies and the opportunities afforded by the COVID-19 pandemic have spurred investment in the life sciences outsourcing sector, according to a panel of industry experts speaking at CPHI North America on Monday. -
Whitepaper Drug Formulation Development Process Notes From A CDMO
Pharma R&D productivity has remained stagnant in the new millennium, despite record industry growth. While new technology has improved productivity in nearly every other sector, efficiency in pharmaceutical R&D is declining (or as described by MedCity News — “broken”).What obstacles are in the way of improving productivity in the pharmaceutical industry? How can we meet these challenges? At Ascendia Pharma, we’re proud to have innovative strategies in place to help our clients get their drugs from R&D to market faster. -
News 4 Factors Affecting Solubility of Drugs
Bringing a new drug—or a new formula—to market is an exciting time for pharmaceutical companies, but getting the formulation correct can be challenging. Low aqueous solubility is a top concern encountered with formulation development of new chemical entities (NCEs). Improper drug solubility can lead to suboptimal drug delivery and absorption, resulting in ineffective drug efficacy and side effects. That's why solubility of drugs must be evaluated in the early stages of drug discovery. -
News Formulation Forum: Nanosuspension Dosage Forms
Nano-formulation of poorly water-soluble drugs has brought two proven benefits to commercial drug products. One is it enhances drug dissolution and oral bioavailability. Second, nano-formulation increases drug loading and release duration for parenteral drug delivery. In the June issue of issue of Drug Development & Delivery, Ascendia Pharma CEO Jim Huang, Ph.D., discusses one method of preparing nanosuspensions in his Formulation Forum column.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance