Magnesium Lactate Trihydrate
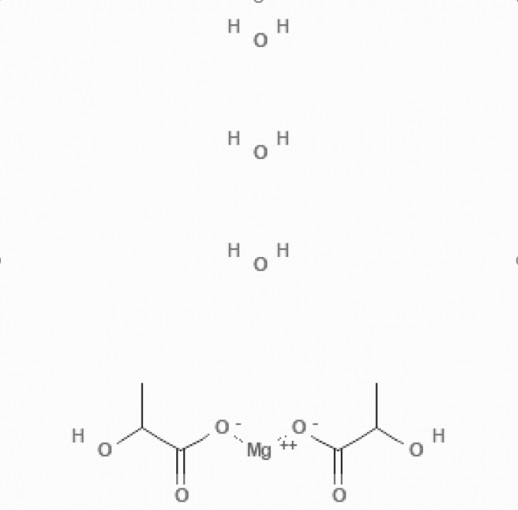
Product Description
Hovione Farmaciencia S.A

-
PT
-
2015On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Categories
Specifications
Hovione Farmaciencia S.A

-
PT
-
2015On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
More Products from Hovione Farmaciencia S.A (43)
-
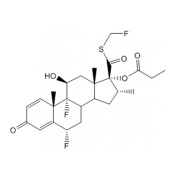
Product Fluticasone Propionate
Hovione has a proven track record in developing and manufacturing high performance APIs for the most demanding applications such as inhalation.
With an unrivalled range of particle size reduction technologies for the production of tailor made API, featuring a customizable, near-perfect partic... -
Product Glycopyrronium Bromide
Hovione’s inhalation grade Glycopyrronium bromide (also known as Glycopyrrolate) particles are highly stable when exposed to high relative humidity and have zero amorphous content.
This highly crystalline micronized and stable Glycopyrrolate can only achieved thanks to Hovione’s extensive in... -
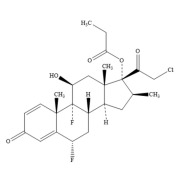
Product Halobetasol Propionate
Hovione has over 57 years of experience in the manufacture of corticosteroids with uncompromising quality and an unblemished Regulatory track record. ROUTE OF ADMINISTRATION: Topical
REGULATORY STATUS: US DMF -
Product Hydrocortisone Aceponate
CorticosteroidsROUTE OF ADMINISTRATION: Topical
REGULATORY STATUS: ASMF & VMF -
Product Indacaterol Maleate
Indacaterol for development trials with customized Particle Design, resulting in Inhalation grade material with highly customizable PSD to befit your application.
Our technology and expertise range yields unmatched impurity profiles with highly reproducibility between batches, resulting in a s... -
Product Ivermectin Human grade
The peace of mind of supply of a stable supplier, founding member of Rx-360 consortium. Hovione Ivermectin has high quality and we offer full regulatory support for human applications
ROUTE OF ADMINISTRATION: Oral, Topical
REGULATORY STATUS: CEP, US DMF, VMF
... -
Product Minocycline Base
Antibiotics
ROUTE OF ADMINISTRATION: Oral, Injectable
REGULATORY STATUS: US DMF -
Product Minocycline Hydrochloride
ROUTE OF ADMINISTRATION: Oral, Injectable, Topical
REGULATORY STATUS: CEP, US DMF, JP DMF
-
Product Mometasone Furoate Anhydrous
High performance APIs for inhalation as well as other demanding applications. The diversity and experience in particle size reduction technologies provides our Customers with tailored made, highly reproducible particle size distributions. Low amorphous content and high stability w... -
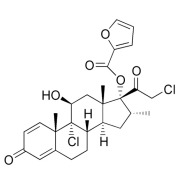
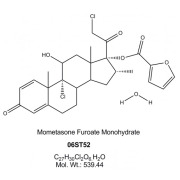
Product Mometasone Furoate Monohydrate
Proven expertise in development of high performance APIs for demanding applications such as inhalation or nasal suspensions. The range of particle size reduction technologies enables the manufacture of tailor made material with high particle size distribution reproducibility across batches a... -
Product Olodaterol Sulphate
LABA/LAMAROUTE OF ADMINISTRATION: Inhalation
REGULATORY STATUS: DEV
-
Product Omadacycline
ROUTE OF ADMINISTRATION: Oral, Injectable
REGULATORY STATUS: DEV
Hovione Farmaciencia S.A resources (24)
-
News Hovione to create new campus and 100 jobs at New Jersey site
The new 31,000-sq. ft building will bring additional commercial spray drying capacity for customers who favour US-based manufacturing -
News Hovione Announces the Appointment of Jean-Luc Herbeaux as Chief Operating Officer
Hovione announced today the appointment of Dr. Jean-Luc Herbeaux as Chief Operating Officer (COO), effective May 1st, 2020. -
News Hovione Announces Partnership to Support Manufacturing of Antiviral Veklury® for COVID-19
Hovione announced the signing of a partnership agreement with Ligand to significantly ramp up the production output of Captisol® -
News Hovione Technology unveils collaboration for new asthma product in Turkey
Ventofor Combi Fix delivered by the PowdAir Plus dry powder inhaler. -
News Hovione's 'world's first' innovative inhaler receives prestigious design award
The 8Shot DPI is capable of delivering high doses of drug to the lungs. -
Video HOVIONE Our Purpose
This sun, this sea is what inspired the Portuguese to be the first to sail around the world five centuries ago. Today, discovery still requires courage, a willingness to take risk, and the best technologies. At Hovione this is what we do. We give clients what they cannot find elsewhere, we do well what is difficult.
We work to help develop new and better medicines. Chemistry, processing and mathematical models are subjects we master, but it all begins and ends with the people. The almost 2000 committed, creative, rigorous, highly qualified team members coming from all parts of the world in which we operate. To contribute to their talent development is one of our major challenges and achievements. -
Video ASD-HIPROS – A new platform for quick and effective formulation screening for Amorphous Solid Dispersions by Spray Drying.
Introduction to Hovione’s new formulation screening platform by Spray Drying Learn about the advantages of a platform that integrates in silico computational models, formulation, analytical development Get to know more about Hovione’s extensive expertise in Spray Drying This session was originally broadcast live as part of CPHI North America 2021 -
Video The Coming of Age of Amorphous Solid Dispersions
The Coming of Age of Amorphous Solid Dispersions
In recent years, numerous drugs formulated as amorphous solid dispersions (ASDs) have been successfully commercialized, confirming the effectiveness of these technologies. Get to learn more with this scientific article. During the 10 days’ event meet with Hovione’s experts safely online and find how our experienced multi-disciplinary teams and their innovative work can help your project succeed. -
Video SCIENTIFIC ARTICLE: The Coming of Age of Amorphous Solid Dispersions
READ ARTICLE The Coming of Age of Amorphous Solid Dispersions In recent years, numerous drugs formulated as amorphous solid dispersions (ASDs) have been successfully commercialized, confirming the effectiveness of these technologies. Get to learn more with this scientific article. During the 10 days’ event meet with Hovione’s experts safely online and find how our experienced multi-disciplinary teams and their innovative work can help your project succeed. -
Video VIRTUAL TOUR Welcome to one of our state-of-the-art facilities
WATCH VIDEO For more than 60 years, Hovione has been dedicated to improving the lives of millions of patients, collaborating with our clients to create great medicines. In a time we need to adapt to new – and unprecedented – challenging circumstances, all our doors are open with virtual tours and Hovione team members are working enthusiastically with our partners, to solve complex problems and deliver solutions. Get to know more about us: During the 10 days’ event meet with Hovione’s experts safely online.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













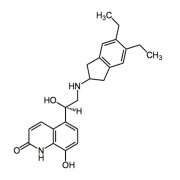
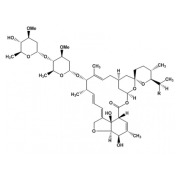
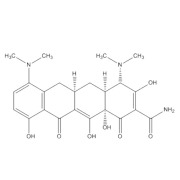
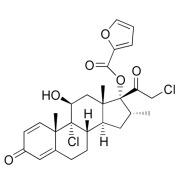








.jpg)










-file120176.png)



