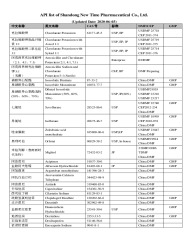
Isosorbide dinitrate

Product Description
Shandong Newtime pharmaceutical Co., Ltd.
-
CN
-
2015On CPHI since
-
1Certificates
Company types
Specifications
Shandong Newtime pharmaceutical Co., Ltd.
-
CN
-
2015On CPHI since
-
1Certificates
Company types
More Products from Shandong Newtime pharmaceutical Co., Ltd. (25)
-
Product Orlistat
Orlistat
Production capacity: 50 tons
Quality standard: enterprise standard (≥98.0%); USP (98.0%~101.5%)
Certificate: USDMF29914
Orlistat is a therapeutic drug for obesity that does not act on the central nervous system. It acts ... -
Product Miglitol
Miglitol
Production capacity: 20 tons
Quality standard: Enterprise standard (98.0%~102.0%)
Certificate: 228MF10006/PMDA
Appearance and traits: white to light yellow crystal powder
classification
Primary classification: Endo... -
Product DAUNORUBICIN HYDROCHLORIDE
Daunorubicin HCl
Cas No.: 23541-50-6
Chemical Name:
10-[(3-Amino-2,3,6-trideoxy-indole-L-lysylpyranyl)oxy]-7,8,9,10-tetrahydro-6,8,11- Hydrochloride of trihydroxy-8-acetyl-1-methoxy-5,12-naphthalenedione (8S-10S)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy -ɑ-L-l... -
Product Epirubicin Hydrochloride
Epirubicin HCl
Traits:
This product is red or orange red powder
Quality standard: EP
Storage: shading, sealed, kept in a cool dry place.
Chemical Name:
(8S,10S)-10-[(3-Amino-2,3,6-tripleoxy-α-L-arabinopyranosyl)oxy]-6... -
Product Montelukast Sodium
Montelukast Sodium
CAS No.: 151767-02-1
Appearance: white or off-white hygroscopic powder
Packing: 1KG 5KG 25KG UN cardboard drum
Storage: Protect from light, seal, and store at room temperature
Uses: anti-asthma drug subs... -
Product ZOLEDRONIC ACID
Zoledronic Acid
Cas No.: 165800-06-6
Chemical Name
[1-hydroxy-2-(1-imidazolyl)ethylidene diphosphate monohydrate
[1-Hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl]bis-(phosphonic acid) monohydrate
Storage
Sealed and stored at ... -
Product DILUTED ISOSORBIDE MONONITRATE (50%)
Appearance: A white crystalline powder
-
Product DILUTED ISOSORBIDE MONONITRATE (60%)
• Appearance: A white powder. • Diluted Isosorbide Mononitrate (60%) conforms to USP standards, USDMF 23216, CEP DMF preparing, in compliance with US cGMP.
-
Product DILUTED ISOSORBIDE MONONITRATE (70%)
• Appearance: A white powder.
• Diluted Isosorbide Mononitrate (70%) conforms to USP standards, USDMF 23217, CEP DMF preparing, US cGMP.
-
Product DAPTOMYCIN
Appearance: A pale yellow to pale brown powder, hygroscopic.
-
Product FAROPENEM SODIUM
Appearance: White to light yellow, crystals or crystalline powder.
-
Product Rosuvastatin Calcium
Rosuvastatin Calcium
Cas No.: 147098-20-2
chemical name:
Bis-[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-pyrimidin-5-yl](3R,5S )-3,5-dihydroxyhept-6-enoic acid] calcium salt (2:1)
Calcium bis[(3R,5S,6E)-7-[4-(4-fluorophe...
Shandong Newtime pharmaceutical Co., Ltd. resources (8)
-
News Zhang Guimin was Awarded the Title of “Linyi Municipal Meritorious Entrepreneur”
New Media Department Yu Jie, Duan Yuqing April 22, 2021
On April 21t, the meeting of the summary and commendation of the comprehensive assessment of the economic and social development of Linyi City in 2020 and the promotion of high-quality development in 2021 was held. Wang Ande, secretary of the Linyi Municipal Party Committee, attended and delivered a speech. Meng Qingbin, deputy secretary of... -
News Lunan Pharma in International Platform
New Media Department Yu Jie, Duan Yuqing April 21, 2021
Lunan Pharmaceutical has established an international drug research and development center, which is in line with the international standards, and established a quality assurance system that is in line with the international advanced quality supervision concepts such as FDA, ICH and EDQM. 120 scientific and technological achi... -
Brochure Introduction of Shandong New Time Pharmaceutical Ltd
Shandong New Time Pharmaceutical Co., Ltd. is a subsidiary company of Lunan Pharmaceutical Group Corporation- a national key high and new technology enterprise. It is the largest, comprehensive, modernized, garden-like pharmaceutical production base in China. It mainly focuses on the developing and producing of the raw materials and dosages of the traditional Chinese medicine, chemical medicine and bio products. All its production area passed the GMP authentication. The main products include Clavulanate Potassium(300tons per year), Isoflurane (150tons per year), Sevoflurane(260tons per year), Faropenem sodium (10tons per year), Orlistat(140tons per year), Montelukast sodium(10tons per year), Zoledronic acid(100kg per year), Isosorbide Dinitrate, Miglitol, Acipimox, Alfuzosin Hydrochloride, Daunorubicin Hydrochloride, Gimeracil, Mosapride Citrate, Mycophenolate Mofetil,Tolterodine Tartrate, Hexafluroisopropanol, TBA
-
Brochure Introduction of Lunan Better Pharmaceutical Co., Ltd.
Lunan Better Pharmaceutical Co., Ltd was constructed and put into operation in August, 1992. It is also a subsidiary company of Lunan Pharmaceutical Group Corporation.
-
Brochure Clavulanate potassium mixture
API, FINISHED PRODUCTS, Traditional Chinese Medicine, Biosimilar Products:
Clavulanate potassium with Microcrystalline Cellulose 1:1;Clavulanate Potassium with Silicon Dioxide 1:1; Amoxicillin and Clavulanate Potassium (2:1, 4:1, 7:1) ;Amoxicillin Sodium and Clavulanate Potassium 5:1 (Sterile) -
Brochure Orlistat
Orlistat API, USP standard and In-house standard. We can do CMO and CDMO if you needed.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance