Brochure
21 Sep 2020
Sterile Fill-Finish Development & Manufacturing Brochure

PDF 1001 kB
Content provided by our supplier
Alcami

-
US
-
2016On CPHI since
-
1000 - 4999Employees
Company types
Other Content from Alcami (17)
-
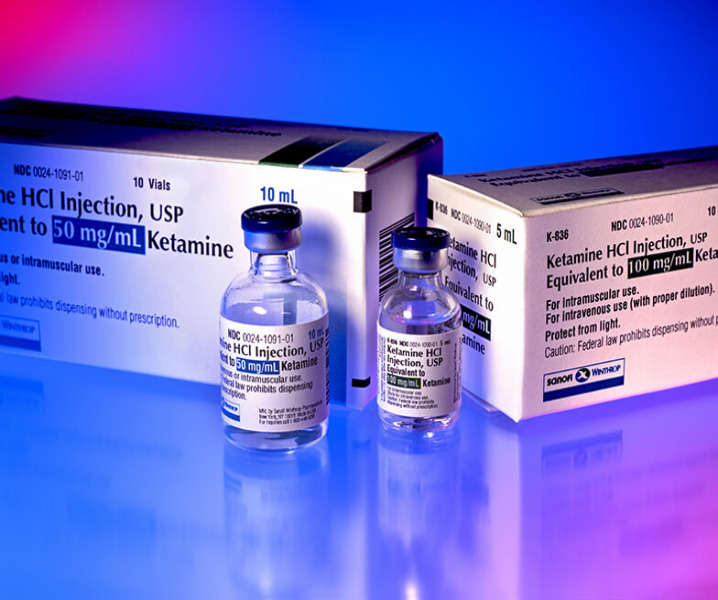
News Alcami to manufacture PharmaTher’s proprietary ketamine products
The CDMO will supply expertise in GMP sterile fill-finish manufacturing and controlled-substances -
Brochure Food Studies Brochure
Alcami is equipped and ready to handle study design and execution of food studies to support a variety of oral solid dosage forms, including tablets, capsules, and suspensions. Our subject matter experts have developed and executed studies using infant formula, yogurt, orange juice, apple sauce, smoothies, and a variety of other foods and drinks. -
News RSVP for Formulation & Sterile Fill-Finish Online Open House!
Sign-up below to be part of an open house with key members of Alcami's operational and commercial leadership teams for sterile fill-finish manufacturing services on October 9. -
News Jacque Uribe and Elliott Franco Join Line-Up for Open House
Join us this Friday for the opening of our new formulation and sterile fill-finish facility with a digital open house from 1:00 to 2:00 pm EDT. Join key members of Alcami's operational and commercial leadership teams to learn about our newest facility featuring four filling lines with isolator and flexible manufacturing technology. RSVP below. -
Whitepaper Stability eBook
Alcami scientific experts go back to the basics to examine the importance of stability in pharmaceuticals. -
News New Formulation & Sterile-Fill Finish Facility Online Open House on Oct 9
We’re kicking off the opening of our new, 32,000 sq. ft formulation and sterile fill-fill finish facility with a digital open house on October 9 from 1:00-2:00 pm EDT. We have a range of technical experts as our speakers including Maria Lacourt, Ken Domagalski, Jacque Uribe, Yadira Salamander, and Kim McClintock. Join us! -
Video Alcami RTP Laboratory Video
Enjoy a look inside our brand new laboratory in Research Triangle Park, North Carolina. This state-of-the-art facility houses formulation development, chemistry, microbiology, and environmental monitoring capabilities in support of our also brand new formulation and new sterile-fill finish facility in RTP. -
Video Expanding the OSD toolbox with an industry-based approach to lipid and surfactant adsorption onto dry powder excipients to increase the dissolution rate of BCS Class II and Class IV active pharmaceutical ingredients (API’s)
Common high-performance lipids and surfactants were used to improve the dissolution rate of furosemide as a model compound for BCS Class II and Class IV active pharmaceutical ingredients. Furosemide equilibrium solubility was measured throughout an HLB range (1-16) of liquid lipids and surfactants. Furosemide tablets were then manufactured using high shear granulation to absorb the solubilized and suspended furosemide onto microcrystalline cellulose (Avicel PH101). The granulations were then compressed using a rotary tablet press and standard tablet tooling. Finished tablets were analyzed to compare dissolution rates using 0.1 N HCl media and USP Apparatus II. Higher equilibrium solubility yielded increased dissolution rates for furosemide tablets compared to directly compressed furosemide tablets. -
Webinar Expanding the OSD Toolbox with an Industry-Based Approach to Lipid and Surfactant Adsorption onto Dry Powder Excipients to Increase the Dissolution Rate of BCS Class II and Class IV Active Pharmaceutical Ingredients (API’s)
Common high-performance lipids and surfactants were used to improve the dissolution rate of furosemide as a model compound for BCS Class II and Class IV active pharmaceutical ingredients. Furosemide equilibrium solubility was measured throughout an HLB range (1-16) of liquid lipids and surfactants. Furosemide tablets were then manufactured using high shear granulation to absorb the solubilized and suspended furosemide onto microcrystalline cellulose (Avicel PH101). The granulations were then compressed using a rotary tablet press and standard tablet tooling. Finished tablets were analyzed to compare dissolution rates using 0.1 N HCl media and USP Apparatus II. Higher equilibrium solubility yielded increased dissolution rates for furosemide tablets compared to directly compressed furosemide tablets. -
Whitepaper The Changing R&D Landscape: Emerging Technology Needs and Value Proposition for Strategic Partnerships with Contract Development and Manufacturing Organizations (CDMOs)
We expect the importance and value proposition of manufacturing to exponentially increase in the next decade and the foreseeable future. Most importantly, parenteral manufacturers need to move away from a myopic "fill-finish" mentality to a fully integrated development mindset that is in line with client expectations, patient needs, and regulatory requirements. It will require "out of the box" thinking and strategic, at-risk investments to remain ahead of the curve. This will take innovation, courage, and collaboration across R&D and placing smart bets for a win-win. -
Whitepaper Structural Characterization of Biologics Using High-Resolution Mass Spectrometry
High-resolution mass spectrometry is a core technique for the characterization of biologics. It can provide a full range of characterization capabilities ranging from high-level analysis through intact mass analysis to residue-specific information from MS/MS sequencing. Alcami offers a full range of capabilities for the structural characterization of biologics using high-resolution mass spectrometry. Instrumentation includes two high-resolution quadruple time of flight (Qtof) instruments and multiple high sensitivity triple-quadrupole instruments. -
Video Video of Alcami's Newest Laboratory Facility in RTP, North Carolina
Enjoy a look inside our brand new laboratory in Research Triangle Park, North Carolina. This state-of-the-art facility houses formulation development, chemistry, microbiology, and environmental monitoring capabilities in support of our also brand new formulation and new sterile-fill finish facility in RTP. -
Whitepaper Design and Execution of Food Studies
Alternate methods of administering solid oral formulations are necessary to meet the needs of all patients. Geriatric, pediatric, and patients suffering from gastroesophageal reflux disease are often administered pharmaceuticals mixed with food or liquids due to difficulty swallowing tablets or capsules. For immediate release, enteric coated, modified release, or extended release dosage forms, a food study is required to evaluate whether the alternate route of administration impacts the therapeutic dose.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















-file148145.png)
-file148399.png)



-file108985.jpg)