Heron (Shanghai) Pharmaceutical Science & Technology Co., Ltd.
About Heron (Shanghai) Pharmaceutical Science & Technology Co., Ltd.
Categories

-
CN
-
2019On CPHI since
-
1 - 24Employees
Company types
Meet us at
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Products from Heron (Shanghai) Pharmaceutical Science & Technology Co., Ltd. (9)
-
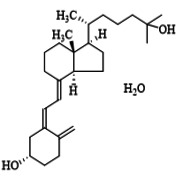
Product Calcifediol
in compliance with the monographs of USP/EP/BP, produced under GMP conditions, indicated for Osteoporosis, Rickets and Osteomalacia. -
Product Maxacalcitol
produced under GMP conditions, indicated for Secondary hyperparathyroidism and Psoriasis -
Product Calcitriol
Calcitriol (骨化三醇) in compliance with BP/USP/EP monographs, produced under GMP conditions, indicated for Rickets, Osteoporosis, Hypoparathyroidism, Renal osteodystrophy and Osteomalacia. -
Product Alfacalcidol
Alfacalcidol (阿法骨化醇) is in compliance with the monographs of EP/BP/ChP Grade, produced under GMP conditions and indicated for Osteoporosis, Chronic renal insufficiency, Hypoparathyroidism, Vitamin D-resistant rickets and Osteomalacia. -
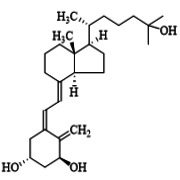
Product Calcipotriol monohydrate
EP/BP Grade, produced under GMP conditions, indicated for Psoriasis Vulgaris. -
Product Doxercalciferol
in compliance with Monograph of USP, produced under GMP conditions and indicated for Secondary hyperparathyroidism -
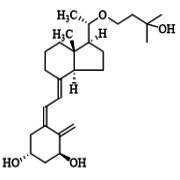
Product Eldecalcitol
Produced under GMP conditions, indicated for Osteoporosis -
Product Paricalcitol
USP grade, produced under GMP conditions and indicated for Secondary hyperparathyroidism -
Product Tacalcitol Monohydrate
in compliance with the monographs of EP/JP, produced under GMP conditions and indicated for Psoriasis Vulgaris
Heron (Shanghai) Pharmaceutical Science & Technology Co., Ltd. Resources (3)
-
Video Heron Pharma Interview
Heron Pharmaceutical Science & Technology Co., Ltd. was founded in 2013 and grew fast in the past 6 years. Chuangye Jiangsu 8090+ interviewed Heron Pharma on July 4th, 2019 since this company is one successful case of New Born Company in Nanjing City. This video could help more people to understand Heron Pharma and know more interest details how we work.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance