Grifols International, S.A.
About Grifols International, S.A.
Certifications
Categories

-
ES
-
2015On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
Meet us at
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from Grifols International, S.A. (4)
-
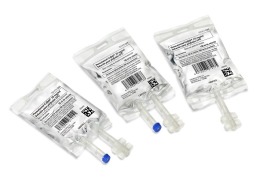
Product Pre-mixed IV solutions: bags
Premixed solutions in customized IV bags, sizes: 50, 100, 250, 500 and 1000 ml (one port, two ports, twist-off, luer valve)
Grifols Partnership is specialized in developing and manufacturing high quality sterile solutions in polypropylene flexible bags in different sizes to suit every need in both ... -
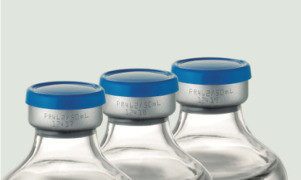
Product Pre-mixed IV solutions: vials and bottles
Glass vials (5mL-50mL) Glass bottles (50mL-500mL)
Grifols Partnership is specialized in developing and manufacturing high quality sterile solutions in glass vials and bottles to suit every need in both the human and veterinary markets. -
Product Water for Injection
Because diluent is as important as your API, Grifols offers you Water for Injection Drug Master File (DMF) -
Product Sodium Chloride 0,9% USP
0.9% Sodium Chloride Injection USP in Fleboflex® plastic container
Grifols International, S.A. Resources (15)
-
Brochure Grifols Partnership general brochure
Detailed information about Grifols Partnership's services, products and capabilities, offered to pharmaceutical companies. -
Video See Grifols sites around the world from the sky!
Have you ever wondered what Grifols sites around the world are like? This aerial tour video provides a great visual perspective of various sites. Take two and a half minutes to watch it and see our sites around the world.
-
Whitepaper A word with... Carla Mössinger and Marga Viñes
In this exclusive interview, Marga Viñes, Senior Business Development Manager for
Contract Manufacturing and Carla Mössinger, Business Development Manager for IV Solutions & Contract Manufacturing answer questions about the quality, flexibility, and efficiency of Grifols Partnership CDMO services. -
Video What do our customers say about us?
Our customers are happy customers! See what they say about our contract manufacturing services and what is like to trust their project in us! -
Video Back to the Future of Healthcare
Michael Howell, Site Director of Albumin Manufacturing at Grifols, speaks with Nigel Walker about how Grifols’ state-of-the-art facility in Dublin meets client demands in this new era in healthcare. They also discuss how risk assessment plays a major role in Grifols' success with customers. -
Video A CDMO Dedicated to Supporting Small-Volume, Small-Molecule Parenteral Projects
Watch Pharma’s Almanac exclusive interview with Oriol Prat, Oriol Argemí and Marga Viñes, about trends in parenteral manufacturing, and how Grifols is maintaining its leadership in the small-volume parenteral manufacturing market. -
Whitepaper Catering to a growing demand for Small-volume parenteral manufacturing
Drug candidates are becoming increasingly complex as manufacturers continue to focus on niche and personalized medicines. Contract development and manufacturing organizations with process and capacity, flexibility, and wide-ranging expertise in small-volume GMP manufacturing are needed to support efforts by pharma companies to rapidly bring these specialized products to the market. -
Whitepaper The critical role of continous improvement in parenteral drug manufacturing
In the competitive pharmaceutical manufacturing landscape, the ability to continuously achieve improvements in efficiency and productivity is essential to success. Given the higher complexity of parenteral drug manufacturing, continuously improving production processes is also paramount for ensuring the highest level of quality. -
Whitepaper Investing in Form-Fill-Seal technology
Extensive process understanding is required to ensure the consistent manufacture of high-quality sterile parenteral products. At Grifols, our use of state-of-the-art automated aseptic processing systems, including form-fill-seal (FFS) technology, ensures control with minimal human intervention. -
Whitepaper A CDMO Dedicated to Supporting Small-Volume, Small-Molecule Parenteral Projects
Read Pharma’s Almanac exclusive interview with Oriol Prat, Oriol Argemí and Marga Viñes, regarding trends in parenteral manufacturing, and how Grifols is maintaining its leadership in the small-volume parenteral manufacturing market. -
Video Grifols Partnership CDMO services
Combining the focus on parenteral with the expertise of an established pharmaceutical leader, the contract manufacturing business is agile and flexible in its service to companies outsourcing the development and manufacture of added value injectable drugs. -
Brochure Grifols Partnership flyer
Discover Grifols Partnership's services, products and capabilities offered to pharmaceutical companies. -
Brochure 0.9% Sodium Chloride Injection USP Fleboflex®
0.9% Sodium Chloride Injection, USP, in Fleboflex® plastic container. Delivering a safe, reliable, and convenient IV solution. -
Brochure Grifols Partnership vials brochure
Grifols Partnership, your CDMO for filling vials. Because diluent is as important as your API. -
Video What do our customers say about us?
Watch Neil Owens' interview, President and Chief Operations Officer at Medicure Inc about their experience working with Grifols Partnership.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance