GENOVIOR BIOTECH CORPORATION
About GENOVIOR BIOTECH CORPORATION
Certifications
Categories

-
TW
-
2016On CPHI since
-
1Certificates
-
100 - 249Employees
Company types
Primary activities
Meet us at
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
CPHI South East Asia 2025
MITEC, Kuala Lumpur, Malaysia
16 Jul 2025 - 18 Jul 2025
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from GENOVIOR BIOTECH CORPORATION (9)
-
Product Semaglutide (GLP-1 Drug)
API < USD 500 per gram, COA available Finished product -
Product CDMO Services
Genovior is a dedicated CMO/CDMO, focusing on providing clients excellent, efficient, one stop services and solutions, from clinical trials (IND/NDA) to commercialization.
Our services include:
1. Process Development
2. Formulation Development
3. Analytical Development
... -
Product CDMO Services - API
small moleculepeptide
biologics -
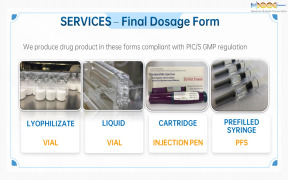
Product CDMO Services - FDF drugs
VialsPrefilled Syringe
Injection Pen -
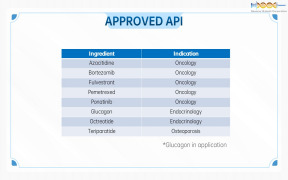
Product Genovior Products - API
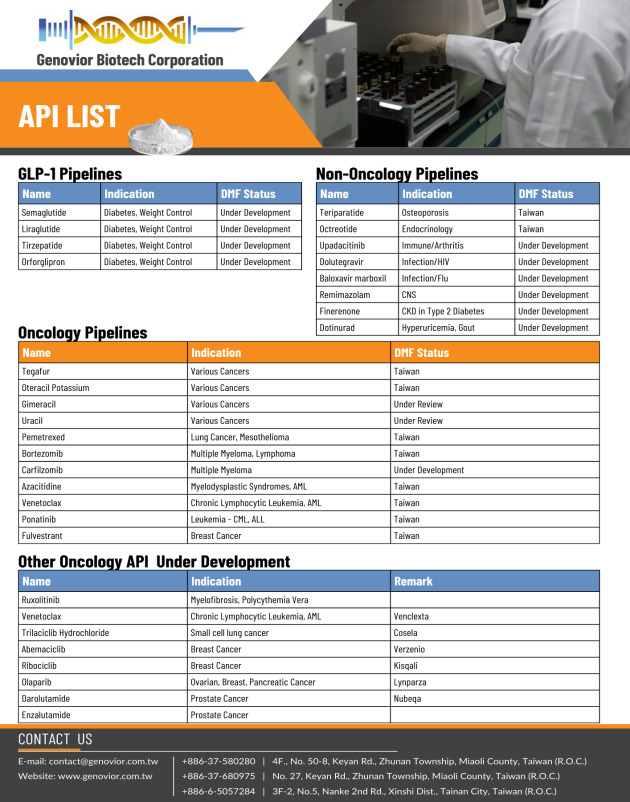
• Azacitidine (Oncology) • Bortezomib (Oncology) • Fulvestrant (Oncology) • Pemetrexed (Oncology) • Ponatinib (Oncology) • Glucagon (Endocrinology) • Octreotide (Endocrinology) • Teriparatide (Osteoporosis)
-
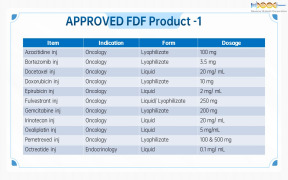
Product Genovior Products - FDF Drugs List 1
Azacitidine inj
Bortezomib inj
Docetaxel inj
Doxorubicin inj
Epirubicin inj
Fulvestrant inj
Gemcitabine inj
... -
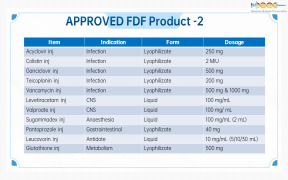
Product Genovior Products - FDF Drugs List 2
Acyclovir inj
Colistin inj
Ganciclovir inj
Teicoplanin inj
Vancomycin inj
Levetiracetam inj
Valproate inj
... -
Product Interview by Newsweek, time to talk
Interviewee:Dr. Steve J.P. Hsu (President of Genovior Biotech) -
Product Products & Service
Products & Service
GENOVIOR BIOTECH CORPORATION Resources (9)
-
Brochure COMPANY PRESENTATION
Genovior is a dedicated CMO/CDMO, focusing on providing clients excellent, efficient, one stop services and solutions, from clinical trials (IND/NDA) to commercialization.
Our services include:
1. Process Development
2. Formulation Development
3. Analytical Development
4. Small Scale Biologics / Injectable Production
5. Commercial Scale Injectable Production
6. Dedicated Oncology Production (API / Injectables)
7. Regulatory Affairs
Generic/Peptide/Biosimilar projects available for out-licensing
Oncology-Azacitidine, Bortezomib, Pemetrexed, Fulvestrant
Peptide-Glucagon, Teriparatide, Octreotide, Terlipressin
Biosimilar-Liraglutide, Ranibizumab, Denosumab
Others-Levetiracetam, Palonosetron, Valproate
-
Brochure Interview by Newsweek, Time to Talk
Interviewee:Dr. Steve J. P. Hsu (President of Genovior Biotech)
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance