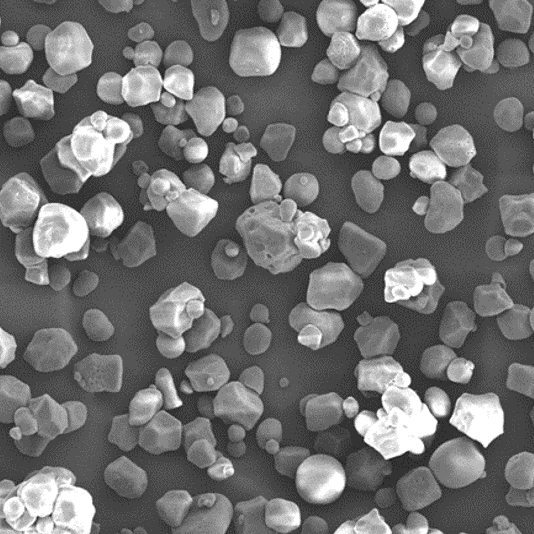
Geleol Mono and Diglycerides NF

Product Description
Gattefosse S.A.

-
FR
-
2015On CPHI since
Company types
Categories
Gattefosse S.A.

-
FR
-
2015On CPHI since
Company types
More Products from Gattefosse S.A. (46)
-
Product Maisine CC
Maisine® CC (glyceryl monolinoleate) is an oil composed of long-chain mono-, di-, and triglycerides for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble lipophilic APIs. Used in self-emulsifying lipid formulations (SEDDS and SMEDDS) as an oily vehicle. Its hi... -
Product Compritol 888 ATO
Compritol® 888 ATO (glyceryl dibehenate) is a glyceride with a high melting point used as a modified release agent or lubricant in tablets. Can also be used in lipid coating technologies and as lipid carrier for nanoparticles.
-
Product Compritol 888 Pellets
Compritol® 888 Pellets (glyceryl dibehenate) is a glyceride with a high melting point used as consistency agent in topical formulations.
-
Product Compritol E ATO
Compritol® E ATO (for nutraceuticals) is a high melting point lipid obtained by the esterification of glycerin with C22 fatty acid, conforming to food additive status. The powder form is used as a modified release agent or lubricant for high-dose nutraceutical tablets.
PALM OIL FREE version a... -
Product Compritol HD5 ATO
Compritol® HD5 ATO (behenoyl polyoxyl-8 glycérides) is a glyceride with an intermediate melting point containing PEG-8 esters. A powder wetting agent and lubricant for effervescent and fast-dissolving tablets.
-
Product Emulcire 61WL 2659
Emulcire 61WL 2659 is a non-ionic oil-in-water emulsifier for use in association with Gelot 64 as a co-emulsifier to improve heat stability in creams. It is available in semi solid pellets form. -
Product Geloil SC
Geloil SC is a mixture of refined soybean oil USP-NF, glyceryl distearate USP-NF and polyglyceryl-3 dioleate USP-NF. A thixotropic suspension vehicle for nutraceutical and pharmaceutical APIs. Ready-to-use in a cold process for thermosensitive actives in soft gel capsule.
-
Product Gelot 64
Gelot™ 64 is a nonionic oil-in-water emulsifier for topical emulsions. It provides excellent emulsion stability with difficult-to-formulate and combination APIs. Provides heat stability and high tolerance over a wide pH range. -
Product Gelucire 48/16 Pellets
Gelucire® 48/16 Pellets is a non-ionic pure surfactant for lipid-based formulation to solubilize and increase oral bioavailability of poorly soluble API. Forms micellar solution in aqueous media.
-
Product Gelucire 43/01
Gelucire® 43/01 is a glyceride with an intermediate melting point used as a matrix agent for sensitive APIs and a viscosity-increasing agent in oral and topical formulations.
-
Product Gelucire 44/14
Gelucire® 44/14 (lauroyl polyoxyl-32 glycerides) is a nonionic water-dispersible surfactant for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble APIs. Self-emulsifies in aqueous media forming a fine dispersion, i.e., microemulsion (SMEDDS).
-
Product Gelucire 50/13
Gelucire® 50/13 (stearoyl polyoxyl-32 glycerides) is a nonionic water-dispersible surfactant for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble APIs. Self-emulsifies in aqueous media forming a fine dispersion, i.e., microemulsion (SMEDDS).
Gattefosse S.A. resources (7)
-
News Gelucire® 59/14, solid surfactant for oral bioavailability enhancement
Discover Gelucire® 59/14, an add-on in Gattefossé Gelucire® range, for oral bioavailability enhancement -
News Boost your drug bioavailability with Labrafac™ MC60
Discover Labrafac™ MC60, glycerol monocaprylocaprate, an oral bioavailability enhancer.
-
Video Emulfree® Duo, for bi-gels: An Innovative Texture
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. Patient adherence to skin treatments Bi-gels: formulating new topical drugs Emulfree® Duo, a ready-to-use excipient designed to make bi-gels Case studies -
News Innovate in bi-gel with Emulfree® Duo
Discover our brand new excipient Emulfree® Duo, a ready-to-use stabilizing agent, enabler of bi-gel, an emerging topical dosage form.
-
Video Boost your Drug Bioavailability with Labrafac™ MC60, Glycerol Monocaprylocaprate
This session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme. This session explores: Using Lipid-based formulations to address oral bioavailability issues Labrafac™ MC60, glycerol monocaprylocaprate, Gattefossé new bioehancer Case study: step-by-step development of a lipid-based formulation of dutasteride with Labrafac™ MC60 Gattefossé technical expertise -
News Lipid-based formulations: winning strategy for oral bioavailability enhancement
Many drugs exhibit poor solubility and/or permeability leading to weak oral bioavailability. To overcome these issues, different technologies have been developed. Among them, lipid-based formulation (LBF) is an option worth considering as it can be used from the very beginning of drug development to product, reducing time to market.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





















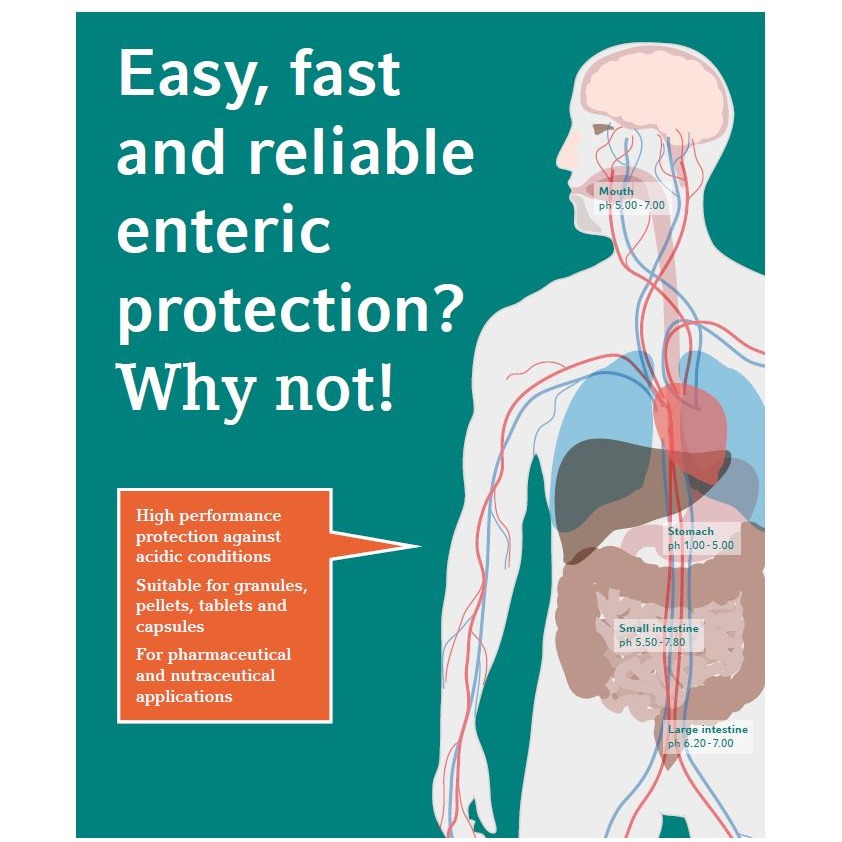






























-comp244983.png)



































![NexArni 50 [Valsartan with Sacubitril Tablets]](/46/product/129/06/57/picture.jpeg)