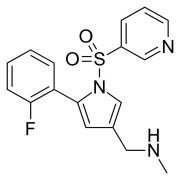
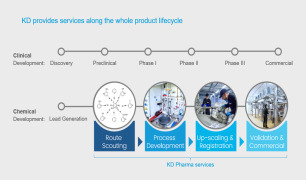
Development Services

Product Description
Adare Pharma Solutions

-
US
-
2015On CPHI since
-
2Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Adare Pharma Solutions

-
US
-
2015On CPHI since
-
2Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Adare Pharma Solutions (13)
-
Product Microcaps® Taste Masking and Modified Release Technology
Microcaps® Taste Masking and Modified Release Technology achieves uniform and efficient coating of drug particles by coacervation (phase separation) to build polymeric membranes of varying porosity and thickness. The result is a free-flowing powder containing microencapsulated API (or API substrate) in a w... -
Product Precision Particle Fabrication™ Technology
Precision Particle Fabrication™ technology produces uniform microspheres and microcapsules with narrow size distribution and precise control over particle structure. The platform technology is flexible and customizable to accommodate a broad range of active ingredients, including small molecules, peptides ... -
Product Optimµm® Technology for Oral Delivery
The Optimµm® technology creates microspheres with high drug loading suitable for taste masking, enteric coatings, and extended release oral applications. The technology is capable of producing single- or double-layer particles in a one manufacturing step, lending to cost-effective creation of palatabl... -
Product Stratµm™ Technology for Injectable Delivery
The Stratµm™ technology, for injectable delivery, allows for precise control of particle size and composition enabling predictable injectable performance, which is critical for self-administration, extended release, and modified release applications with a narrow therapeutic index. Unlike other encapsu... -
Product Unisun® Technology for Otic Delivery
The Unisun® technology combines the use of encapsulated or free drug, along with a fast-film forming agent, which allows for low-cost, long-acting intratympanic delivery of a therapeutic. The technology allows for less frequent administrations than current therapies and is capable of sustaining drug le... -
Product Manufacturing Capabilities
Standard Offerings
• Granulation & Mixing • Fluid Bed Processing & Drying • Hot Melt Extrusion • Pan Coating • Blending • Tableting • Multi-layer Tablets • Capsule Filling • Oven Drying • Small-scale GMP Manufacturing ... -
Product Technologies
Adare’s industry-leading experts possess unparalleled experience in the development of unique dosage forms that provide taste masking, customized release, and patient-centric solutions. Our technology platforms overcome complex formulation challenges to improve the lives of all patients, with expertise in ... -
Product Packaging
Clinical & Commercial Packaging Services
• Two FDA-Registered Packaging Facilities • A Full Range of Solid-Dose Packaging Solutions • High-Speed Bottle Filling • Blister Packaging • Stick Pack & Cartoning Operations • Packaging of DEA Schedules II, 2N, III, 3N, IV, L1 • Thermo &... -
Product Nitrosamine Mitigation
Very few CDMOs can claim Adare's level of experience in mitigating the presence of nitrosamine. We can employ our own-in-house mitigation processes to develop the best long-term control strategies for your product.
Regain control of your product with NitroCLEARx • Receive real-world mitiga... -
Product Adaptdose™
Adaptdose™, Adare’s unique commercialized approach to adaptive formulation design, is a highly flexible, multi-delivery platform tool that eliminates the need for post Phase 1 trial reformulations and facilitates the creation of custom oral delivery systems. Adaptdose provides the ability to combine 2-3 ac... -
Product CAT.one SM
CAT.one SM minimizes the potential for abuse by ensuring that drug products are evaluated for all potential routes of misuse. Adare’s experts integrate abuse-deterrent testing by replicating basic and multi-step methods that an abuser may use to prepare the product for recreational misuse or abuse. Our CAT... -
Product DIFFUTAB® Technology
DIFFUTAB® technology is an effective solution for targeted drug delivery through customized and sustained release. The technology assists the development of high-dosage and sustained release products for once-daily administration. A matrix tablet is coated with functional polymers, followed by a blend of h...
Adare Pharma Solutions resources (22)
-
News Providing solutions for special populations – CPHI North America Interview
In Philadelphia in May on site at CPHI North America we were able to meet with some of our speakers regarding their presentations. In the following interview Srinivasan Shanmugam, Executive Director, Pharmaceutical Sciences, Business Support, and New Technologies at Adare Pharma Solutions discusses his breakfast session. -
Brochure Adare Pharma Solutions Interactive Brochure
Explore Adare's capabilities through our interactive brochure, showcasing our technology-driven CDMO with integrated end-to-end services. With extensive capacity, experience, and a global track record, Adare is your partner for small molecule oral solid dose success.
-
News A Start-Up’s Guide to Choosing Their First CDMO: Breakfast Session Bulletin
Throughout CPHI North America 2023, several Breakfast Sessions were held to start the day off right with a complimentary breakfast and a networking and learning session for our attendees. With insightful presentations from industry experts and partners, the CPHI North America Breakfast Sessions had something for everyone looking to fill their brains and their stomachs. -
Brochure Scientific Briefing - Formulation of Aqueous Core Poly (Lactic Acid) Microcapsules
Poly (lactic acid), or PLA, is a biodegradable, biocompatible polyester commonly used in many medical, pharmaceutical, and biotechnology products. The molecular weight of PLA can be tailored to impart various degradation kinetics and subsequent release profiles. The ability to make microcapsules with a thin PLA shell, however, is non-existent in the controlled release space. Here, using a proprietary Stratμm® technology, we have created 50 μm capsules with an aqueous core and thin PLA shell. The microcapsules have implications for encapsulating water-soluble molecules such as peptides and proteins, which can be released with a discrete pulse, or slow ramp in release rate. -
News CPHI North America 2023 – From the Floor
Follow along for live updates from the Content team as we bring you the latest from CPHI North America 2023 - from session talks, panel discussions, interviews, and more, there's a lot to discover with CPHI Online at the Pennsylvania Convention Center! -
Brochure Scientific Briefing - Formulations of Monodisperse Controlled-Release Quetiapine Microspheres
Adare’s Optimµm® technology offers a unique and efficient method of fabricating monodisperse microparticles at reduced development time. The study herein uses Adare technology to fabricate monodisperse quetiapine microparticles to investigate the effects of formulation and size on the release profile of the API. -
News The Top 5 Industry Content Reads on CPHI Online
If you’re looking for news, product information and market trends from leading pharma companies, the CPHI-Online.com Company Showcases are a great resource for buyers who want to stay up to date, browse product portfolios and find the right partner.The Company Showcase profiles offer a library of free to access, downloadable content, including videos and webinars, reports, whitepapers and product brochures, from the Pharma suppliers and service providers you’d look to meet at our events.
-
Brochure Scientific Briefing - Dissolution Development of Parvulet Technology Oral Tablets
Parvulet® Technology is a patented oral dosage form capable of being dispensed to patients in tablet or powder formats, and converted into a semi-solid at the time of dosage through activation with water. The final dosage is easily administered, as a soft food like texture, ideal for pediatric and geriatric populations (including those with dysphagia). The Parvulet® technology can be combined with our taste-masking and controlled release technologies, providing greater flexibility in advancing patient centric solutions. -
News The Top 5 Industry Content Reads on CPHI Online
If you’re looking for news, product information and market trends from leading pharma companies, the CPHI-Online.com Company Showcases are a great resource for buyers who want to stay up to date, browse product portfolios and find the right partner. The Company Showcase profiles offer a library of free to access, downloadable content, including videos and webinars, reports, whitepapers and product brochures, from the Pharma suppliers and service providers you’d look to meet at our events. -
Brochure Executive Interview with Vice President of Pharmaceutical Development on Growth and Strategy
When Adare Pharma Solutions was acquired in September of 2020 by two private equity firms known for activating growth and innovation, the company sent a signal to organizations that depend on contract development and manufacturing services to bring their pharma solutions to the healthcare market: if you need a trusted partner, now more than ever, Adare is a winning choice. -
News Patient-centric medicines the way forward during COVID-19 crisis, say CPHI panel
Festival of Pharma roundtable discusses how drug delivery formulation and development is focused on hospital-to-home care setting -
Brochure Dedicated Dialogue - Improved Drug Delivery with Precision Particle Fabrication
Precision Particle Fabrication™ technology produces uniform microspheres and microcapsules with narrow size distribution and precise control over particle structure, making the platform versatile enough to accommodate a range of active ingredients and delivery formats. Nathan H. Dormer, PhD, Director of Drug Product Development at Adare Pharma Solutions, speaks about how this new technology platform is expanding the frontiers of therapeutic delivery and improving patients’ experience in the process. -
News The latest formulation approaches for counteracting drug solubility, stability and bioavailability issues
What kind of enhancement solutions do CDMOs need to offer their pharma clients to address pressing issues such as bioavailability, solubility and stability of their products? A recent CPHI webinar sponsored by Adare Pharmaceuticals delved deep into the issue... -
Brochure Case Study - Microcaps Exceeds Stringent Requirements Beyond Taste-Masking
A particularly bitter pediatric macrolide in powder form was brought to Adare for needs beyond just taste masking. The API also needed: fast release due to a narrow absorption window, smooth syrup mouthfeel acceptable to children and infants, over seven days of taste masking after suspension, drug loading suitable for its high dose. How did Adare meet this challenge? -
Brochure Case Study - Stable Microcaps Formulation Masks Bitter HIV Drug for Young Children
Adare Pharma Solutions was asked to create a taste masked, convenient dosage formulation of a very bitter API for patients two-years old and older undergoing treatment of HIV infection. As the drug would be administered in patient home settings, sub-optimal distribution and storage conditions, would need to be also considered as well as flexible dose requirements due to patient to patient weight and age variations. -
Brochure Case Study - Microcaps Outperforms Standard Bead Intermediate Formulations
A particularly bitter pediatric macrolide in powder form was brought to Adare for needs beyond just taste masking. The API also needed: fast release due to a narrow absorption window, smooth syrup mouthfeel acceptable to children and infants, over seven days of taste masking after suspension, drug loading suitable for its high dose. How did Adare meet this challenge? -
Whitepaper How to Achieve Precise and Flexible Dosing with Microparticulates
Adare’s MMTS™ Multi Mini Tablet System platform is the embodiment of what is possible through multiparticulate technology, combining the simplicity and high drug loading capacity of tablets with the flexibility to generate a wide range of release profiles specifically suited to both patient and drug. -
Whitepaper Coacervation - Expanding Solutions for Challenging APIs
A variety of microencapsulation techniques are widely used in the pharmaceutical industry including fluid bed coating, spray drying, solvent evaporation, and more. However, one method—coacervation—offers several unique benefits to developers interested in working with challenging and/or bitter-tasting molecules, managing a product’s lifecycle, and even accelerating development timelines. -
Video Nature's Building Blocks Access to Choice
Access to Choice: Long Acting Injectables for Contraceptives BBC Video -
Video A Guide to Successful Collaboration - Considerations When Choosing Your First CDMO as a Start-Up
In pharma, it is essential to identify the right partner to ensure a successful outcome. Time and time again, mistakes have been made in this process, causing clinical studies to fail and for the company to take a heavy hit. Want to avoid this? join our one-hour masterclass where we will devise a checklist for choosing your first CDMO. Discussion Points Thinking ahead before executing – why it is essential to map out what you need to achieve in all departments Why creating a business partnership case to potential CDMOs will determine if they are the right fit for you A checklist of the common pitfalls and how to avoid -
Webinar Taste Masking of Bitter Drugs For Pediatric Population
This session addresses: Pediatric population and their needs, the significance of taste masking, taste masking strategies, and Adare's taste-masking solutions. This session was broadcast as part of the CPHI North America show. -
Video Patient Centric Dosage Forms: Enhancing Acceptance and Adherence in Special Populations
The following session has been SOLD OUT. For any inquiries, please contact a member of staff on the day Kickstart your CPHI North America with a coffee and pastry, connect with your peers, and listen to exclusive content surrounding patient-centric development The challenge of ensuring medication acceptance and adherence is particularly acute in special populations, such as pediatric and geriatric groups. These populations exhibit distinct pharmacokinetic and pharmacodynamic profiles due to their unique age-related physiological and biopharmaceutical characteristics. Consequently, the standard approaches to medication formulation often fall short of addressing their specific needs. This presentation will explore the unique aspects of pediatric and geriatric populations, highlighting the limitations encountered in the design and development of dosage forms tailored for them, and how those challenges are overcome. A significant focus will be placed on the strategies that can be employed to overcome these challenges, with an emphasis on Adare’s platform technologies. Through this discussion, we aim to shed light on the critical factors that must be considered in the development of effective, patient-friendly pharmaceutical formulations for pediatric and geriatric patients.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
































-file128265.png)









.jpg)






































































-comp271056.png)