Controlled, customized or modified drug release
Controlled, customized or modified drug release Companies (17)
Controlled, customized or modified drug release News
-
News CPHI Pharma Award Winners 2022: Pharmaceutical Packaging & Drug Delivery – Evonik
In this series of interviews, we speak to the teams behind the winning concepts at CPHI Pharma Awards 2022, which was held at CPHI Frankfurt, across the categories from digital innovation to CEO of the year. -
News Adare Pharma acquires oral formulations specialist Frontida BioPharm
The combined company intends to lead in the space of oral formulations, solving customers' complex formulation challenges -
News Patient-centric medicines the way forward during COVID-19 crisis, say CPHI panel
Festival of Pharma roundtable discusses how drug delivery formulation and development is focused on hospital-to-home care setting -
News Eliminating user error from inhaler-based clinical studies
With inhaler use notoriously prone to user error, any clinical study that involves self-administration via an inhaler is challenging from the outset. Help is at hand, however, with a simple-but-smart device developed by Cambridge Design Partnership
Controlled, customized or modified drug release Products (50)
-
Product Packaging
Clinical & Commercial Packaging Services
• Two FDA-Registered Packaging Facilities • A Full Range of Solid-Dose Packaging Solutions • High-Speed Bottle Filling • Blister Packaging • Stick Pack & Cartoning Operations • Packaging of DEA Schedules II, 2N, III, 3N, IV, L1 • Thermo &...
-
Product Liposomal Drug Delivery Technologies Development Support and Analysis
Liposomal drug product characterisation according to the FDA CMC guidance to support your new drug application or biologics license applications. Including:
• Physicochemical parameters determined to be CQAs (e.g. particle size, size distribution, zeta-potential and physical stabi...
-
Product Goserelin 3,6mg (1-Month Implant)
The active ingredient goserelin is a synthetic peptide that can be used primarily in the treatment of hormone-dependent tumors, particularly prostate cancer. Thus, goserelin intake has indirect effects on testosterone levels in men, the lowering of which stops the growth of malignant cancerous tumors. In a...
-
Product Drug Delivery Platforms
Modified release, hot-melt extrusion, ODT, oral thin films, oral sprays, and polymer-based solutions,
Our range of technologies covers solid dosage forms (IR and ER), polymer-based solutions, solid dispersions (hot-melt extrusion), oral sprays, oral thin films, and complex injectables ...
-
Product Celanese Development & Feasibility Lab Services
When you work with Celanese you have access to the right mix of services, support, and proven materials to accelerate your drug delivery program through technical feasibility. The Celanese Development & Feasibility Lab offers formulation development, drug release testing, form factor development, mater...
-
Product CELLETS® – Microcrystalline Cellulose (MCC) Pellets/Spheres, Starter Beads
CELLETS® – MCC spheres or pellets, 100 µm – 1400 µm – are perfect starter beads for multi-particulate dosage forms, tablets, minitablets and capsule applications. CELLETS® are made from 100% certified MCC (Microcrystalline Cellulose) and pure water, only. They are inert, tasteless and odorless. With...
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Emulsification and Encapsulation Technology
On the basis of microfluidic principles, Secoya develops and supplies a novel emulsification technology – RayDrop®- for the production of monodispersed droplets or particles.
The emulsification process in the RayDrop® is based on co-flow and flow-focusing technologies embedded in a ...
-
Product Parenteral drug delivery solutions
Evonik is one of the world’s leading CDMOs for parenteral drug delivery. For complex parenteral drug products designed for systemic, targeted or localized delivery, we are uniquely positioned to serve as a global development partner and solutions provider. Our parenteral drug delivery portfolio includes th...
-
Product Dexlansoprazole Dual Delayed Release Pellets
Available in 22.5% w/w
17 Kg Capacity HDPE Container lined with double polythene bags.
-
Product Soctec® Technology_Self Orienting Capsule Technology
Soctec® was developed for drugs that need to be retained in the stomach for an extended period of time to then being slowly released either for a local effect in the stomach or for absorption in the upper intestine. Soctec® is a versatile extended release gastro-retentive platform technology that ...
-
Product VIP Laser Drill + NIR - Tablet Drilling Machine
A pharmaceutical industry first, the VIP Laser Drill + NIR incorporates optional near-infrared spectroscopy alongside precision CO2 laser drilling and vision inspection for the production of osmotic drugs.
This versatile machine uses NIR inspection to verify a tablet’s enteric coating prior to l...
-
Product XR oral liquids
Extended release oral liquids. Complex NDDS. For general as well as Onco based products.
-
Product Goserelin 10,8mg (3-Month Implant)
The active ingredient goserelin is a synthetic peptide that can be used primarily in the treatment of hormone-dependent tumors, particularly prostate cancer. Thus, goserelin intake has indirect effects on testosterone levels in men, the lowering of which stops the growth of malignant cancerous tumors. In a...
-
Product Nitrosamine Mitigation
Very few CDMOs can claim Adare's level of experience in mitigating the presence of nitrosamine. We can employ our own-in-house mitigation processes to develop the best long-term control strategies for your product.
Regain control of your product with NitroCLEARx • Receive real-world mitiga...
-
Product Technologies
Adare’s industry-leading experts possess unparalleled experience in the development of unique dosage forms that provide taste masking, customized release, and patient-centric solutions. Our technology platforms overcome complex formulation challenges to improve the lives of all patients, with expertise in ...
-
Product Development Services
Comprehensive R&D Services • Preformulation • Formulation Development of Solid Dose Tablets (Capsules, Liquids & Solutions) • Pediatric Formulation • Product Design • Formulation Optimization • Sourcing of APIs & Excipients • Analytical Method Development & Validation • ...
-
Product Manufacturing Capabilities
Standard Offerings
• Granulation & Mixing • Fluid Bed Processing & Drying • Hot Melt Extrusion • Pan Coating • Blending • Tableting • Multi-layer Tablets • Capsule Filling • Oven Drying • Small-scale GMP Manufacturing ...
-
Product Advanced Medical Grade Polymers for Medical Devices
Medical devices have unlimited potential to improve patients’ lives, but there are also immense complexities involved in bringing precision products to the highly regulated healthcare market.
Our advanced portfolio of medical-grade polymers provides the high-quality foundation for a range of optimized...
-
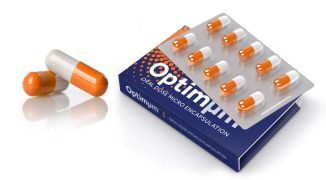
Product Optimµm® Technology for Oral Delivery
The Optimµm® technology creates microspheres with high drug loading suitable for taste masking, enteric coatings, and extended release oral applications. The technology is capable of producing single- or double-layer particles in a one manufacturing step, lending to cost-effective creation of palatabl...
Upcoming Events
-
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



























































.png)







.png)

.png)



.jpg)


