Clinical Supply

Product Description
Recipharm

-
SE
-
2015On CPHI since
-
5000+Employees
Categories
Recipharm

-
SE
-
2015On CPHI since
-
5000+Employees
More Products from Recipharm (21)
-
Product Development & manufacture - drug delivery devices
Bespak by Recipharm delivers market leading design, development, and manufacturing services for drug delivery devices to the global pharmaceutical market. Our portfolio includes inhalers, nasal technologies, and auto-injectors as well as development and manufacturing services. -
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation. -
Product Integrated technology platform
An integrated service for inhalation drug products and devices spanning early-stage development to commercial manufacture. Our depth of knowledge means we can overcome the challenges associated with inhalation drug products and devices. -
Product Drug Product
At Recipharm, we offer drug product development services for all common dosage forms. Our team can manage the complexity of your project, helping you find the best solution whether you are looking for development support to take your product to first-in-human (FIH) studies or to advance your product to mar... -
Product Drug Substance
Recipharm has more than 25 years’ experience in drug substance development and manufacturing. We are ideally placed to deliver a wide range of drug substance services from our dedicated development facilities around the world. -
Product Serialisation
Recipharm have committed to invest in and fully implement the technical solutions needed to comply with global requirements for pharmaceutical serialisation and track and trace. Our serialisation offering includes: • Experience in already providing serialised products to Turkey, Korea and China • Se... -
Product APIs
Recipharm operate facilities which can manufacture several products at a time, handling high pressure, high temperature (up to 150 degrees Celsius) and low temperature (down to –80 degrees Celsius) reactions. Different batch sizes are available ranging from 20L to 6000L, supporting early development and co... -
Product Analytical Development
Recipharm offer analytical support for drug discovery, pharmaceutical development and manufacturing through our global development facilities. -
Product Synthetic Chemistry
Recipharm offer a range of synthetic chemistry services through our global development facilities including advanced lead optimisation medicinal chemistry, process development and tech transfer to large scale GMP-manufacturing and other specialties. -
Product Recipharm Inhalation Solutions™
Recipharm Inhalation Solutions™ offers an end-to-end service for inhalation drug products and devices spanning early stage development to commercial manufacture.Our dedicated team offers pharmaceutical companies a seamless outsourcing service from early stage development through to commercial manufacturing... -
Product Recipharm Analytical Solutions™
Through Recipharm Analytical Solutions™, we support customers with stand-alone analytical requirements. Our analytical development team has experience from developing hundreds of analytical methods every year, supporting development of formulations ranging from powder in capsules and IV solutions to ER tab... -
Product Injectables
Recipharm has extensive experience in the injectables area and supplies small volume parenterals in ampoules and vials, as well as in cartridges. State of the art leak detection and automatic inspection equipment is used and Recipharm’s full service offering is complete with secondary packaging and tempera...
Recipharm resources (47)
-
News Sustainability in pharmaceutical manufacturing: an expert panel discussion
In this written panel discussion experts in manufacturing in the pharmaceutical supply chain comment on the manufacturing landscape, how sustainable practices are being incorporated more readily, the challenges associated with this and the overall advantages. -
Brochure Recipharm's full service offering
Your innovative drug. Our world-class production facilities. Discover the genius of working together. -
News Recipharm receives approval for the manufacture of a new microbiome-based therapeutic
Recipharm receives regulatory approval for the manufacture of a microbiome-based product for the prevention of Clostridioides difficile recurrent infection, under GenIbet Biopharmaceuticals and Seres Therapeutics. -
Brochure Successful drug development in the early clinical phase
Moving from drug discovery into the early clinical development can be both challenging and time consuming without access to the necessary expertise. To perform a phase 1 study successfully, having drug substances and drug products (of the right quality) is crucial. In addition, the right knowledge and resources must also be in place, to ensure the fastest route to clinic. -
News Watch now: Pharma Trends 2022 Webinar
We are approaching the end of a year which saw global vaccine rollout for COVID-19, 44 novel drug approvals (to date) and the return of the CPHI Worldwide in-person event. -
Brochure Exploring new propellants
Over the years, the prominence placed on creating a ‘greener’ future has increased dramatically. As a result, companies globally are facing growing pressure to adopt sustainable processes and reduce their carbon footprint. At Recipharm, we are taking a proactive approach to the situation and will be ready to adapt to any future changes of legislations, as well as supply challenges. -
News Lyophilisation technology improving thermal stability and reducing cycle times, says Recipharm expert
Lyophilisation or freeze drying is an important means to improve thermal stability of a drug product, according to Thomas Becker, Quality Director and Qualified Person, Recipharm. This is particularly true for those that are thermolabile, such as mRNA vaccine candidates.
-
Brochure Development of cannabinoids
At Recipharm, we have the experience and appropriate licencing to support both development and commercial supply of cannabinoids. And, to further assist researchers and pharma companies working in this field, we have also developed new, synthetic routes to five different cannabinoids, including CBDA, CBGA, and three metabolites. In addition, we have identified a novel, scalable, synthetic route to CBD. -
Sponsored Content Key considerations to develop effective analytical methods for your drug project
A summary exploring Recipharm’s key considerations when it comes to developing effective analytical methods for drug projects. -
Brochure Controlled substances
At Recipharm we have been supporting customers with their controlled substance projects for more than 25 years. Our state-of-the-art manufacturing facilities have the infrastructure to safely and securely handle potent materials and we are approved to import, store, manufacture and export Class 1–5 controlled substances. -
News CPHI Podcast Series: Selecting the Right CDMO Outsourcing Partner
With so many outsourcing options available, selecting the CDMO that is the right fit for any particular sponsor company can be a challenging task. -
Datasheet Pilot plant
nhalation development and manufacturing for early and late-stage clinical supply.
Recipharm Inhalation Solutions™ offers comprehensive contract development and manufacturing solutions for early and late-phase clinical supply from our stand-alone pilot plant as part of our integrated inhalation offering.
-
News Sterile manufacturing Annex 1 amendments: what, why and how explained
In January 2020 the European Commission published some proposed significant revisions to Annex 1, the EU’s good manufacturing practice (GMP) guide for the manufacture of sterile products. In the following three-month consultation period, affected organisations and stakeholders from over 70 different countries submitted more than 2,000 comments to the proposed update. -
Brochure Clinical trial material
We understand the need for high quality and timely delivery and work with our customers throughout their development projects to ensure success. Our dedicated development facilities, cleanrooms for cGMP production of solid, liquid, and semi-solid formulations and sterile suites to produce sterile vials, allow us to cater for a range of clinical trials from Phase I to smaller Phase III trials. Large trials will be supplied from our commercial manufacturing sites. -
Sponsored Content CPHI Podcast Series: Selecting the Right CDMO Outsourcing Partner
Sponsors within the biopharmaceutical industry are increasingly turning to outsourcing for several reasons; remaining competitive and flexible within a quickly evolving sector, gaining access to manufacturing capacity and state-of-the-art equipment and scaling up to the commercial level are just three examples. -
Brochure Inhalation device design, development and manufacture
Bespak by Recipharm delivers market leading design, development, and manufacturing services for inhalation drug delivery devices to the global pharmaceutical market, at low, medium and high volume manufacturing scales. -
Sponsored Content Sterile manufacturing Annex 1 amendments: what, why and how explained
In January 2020 the European Commission published some proposed significant revisions to Annex 1, the EU’s good manufacturing practice (GMP) guide for the manufacture of sterile products. In the following three-month consultation period, affected organisations and stakeholders from over 70 different countries submitted more than 2,000 comments to the proposed update. -
Datasheet Innovation services
Bespak by Recipharm delivers market leading design, development, and manufacturing services for drug delivery devices to the global pharmaceutical market. Based in Cambridge, UK, our innovation centre is home to a team with diverse skills and expertise. Established to create new technologies that form the core around which we build and develop reliable, robust products, capable of meeting and exceeding our customers’ expectations with a focus on parenteral, nasal and ophthalmic routes of administration. -
News Recipharm expands its analytical services offering in India
The CDMO inaugurates its new analytical laboratory under Recipharm Analytical Solutions -
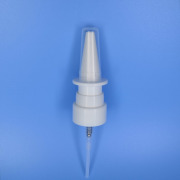
Datasheet Inhalation and nasal product manufacturing
Recipharm offers inhalation and nasal product manufacturing in compliance with FDA and MHRA regulations. We offer contract manufacturing, packaging and supply to the global pharmaceutical market.Our dedicated team has more than 50 years’ experience in manufacturing inhalable products and supports our customers with the development and commercialisation of metered dose inhalers, dry powder inhalers and nasal sprays. -
News Second Curaleaf GMP cannabis facility gains Spanish approval
Medalchemy facility now fully licensed to import, cultivate, extract, manufacture and export medical cannabis flower and extracts -
Datasheet Device contract manufacturing
Bespak by Recipharm delivers market leading design, development, and manufacturing services for drug delivery devices to the global pharmaceutical market, at low, medium and high volume manufacturing scales. We have extensive experience in producing medical devices, including inhalers, nasal technologies, and auto-injectors, for some of the world’s leading pharmaceutical companies. -
News Recipharm selected to operate new fill/finish facility in Morocco
Move is part of US$500 million five-year investment programme by Moroccan government and consortium to establish vaccine and biotherapeutic manufacturing capacity in the country -
Datasheet Extractables and Leachables testing
Our E&L testing capabilities include analytical techniques designed to identify and test a wide range of additives and impurities, which could migrate from packaging to the drug product, potentially affecting drug efficacy and patient safety. -
News Top 5 Industry Content Reads on CPHI Online This Month
If you’re looking for news, product information and market trends from leading pharma companies, the CPHI-Online.com Company Showcases are a great resource for buyers who want to stay up to date, browse product portfolios and find the right partner.
-
Webinar A New Era for the Supply of Life-Saving Medicines: Can BFS Transform the Distribution of Biologics and Vaccines?
Blow-fill-seal (BFS) technology is being explored for one-dose presentations of vaccines and other biologics, providing a continuous, automated process that can lower manufacturing costs, increasing access for patients worldwide. Other BFS benefits for biologics developers include enhanced automated processes with minimal human intervention, lower risk of foreign body contamination, and a high level of container integrity control during fill and finish. Manufacturers also appreciate the minimised risk of breakages during shipping and improved administration usability for clinicians and patients.
However, implementing BFS for biologics and vaccine projects comes with challenges: Ensuring chemical and physical compatibility of the container and the overall blow-fill-seal process with the drug formulation Demonstrating effective drug stability within the container Determining acceptable extractable and leachable profiles. In this session, we will explain how vaccine and biologics developers can overcome these challenges to harness the BFS manufacturing efficiency and patient convenience benefits. -
News Recipharm signs up for aseptic fill-finish manufacturing of Moderna COVID-19 vaccine
The CDMO is already making certain investments to enable technology transfer and scale-up to commence imminently. -
Whitepaper A stability indicating method for analysis of a complex drug product
Find out how Recipharm developed a suitable analysis method for Lecigon® intestinal gel, a combination drug product containing three active pharmaceutical ingredients. -
News Recipharm’s proprietary molecule Erdosteine has been positively tested as part of COVID-19 treatment
Recipharm is pleased to announce that the results of the study with Erdosteine as an add-on treatment for COVID-19 patients are now available. -
Video Soft Mist Inhalers (SMIs) Based on Pre Filled Syringes
Resyca, a joint venture between Recipharm and Medspray, is developing a novel type of soft mist inhaler devices, based on an existing primary packaging: glass pre filled syringes. This way we can utilise existing filling lines within Recipharm to do the aseptical filling. The lecture will describe the devices and the typical timelines for clinical and commercial supply.
Most pharma and biological companies perform their drug discovery, pre clinical and phase 1 clinical work with nebulisers and liquid formulations. By using a soft mist inhaler device, they can take a shortcut and reach the market sooner and with lower development risks. Resyca's soft mist inhalers have been developed with biologics formulations in mind, being able to deliver milligrams of drug in a single inhalation from a pocket size device.
Click here to register
-
News Recipharm to boost sterile liquids manufacturing capacity
The investment will support increased demand and help to secure new business at its Kaysersberg facility. -
Podcast CPHI Podcast Series: Selecting the Right CDMO Outsourcing Partner
Sponsors within the biopharmaceutical industry are increasingly turning to outsourcing for several reasons; remaining competitive and flexible within a quickly evolving sector, gaining access to manufacturing capacity and state-of-the-art equipment and scaling up to the commercial level are just three examples. With so many outsourcing options available, selecting the CDMO that is the right fit for any particular sponsor company can be a challenging task. We speak with Frank Ternes, Chief Commercial Officer and Filip Ringborg, Director Contract Management & Operations Development, at Recipharm on best practice when it comes to selecting the right fit for your outsourcing requirements and how strategic partnerships built on trust are fundamental to success. -
News Recipharm invests EUR 2.6 million in Kaysersberg facility
Global contract development and manufacturing organisation (CDMO), Recipharm, has announced a EUR 2.6 million investment for additional manufacturing capacity to support increased demand into its Kaysersberg facility. -
Webinar CPHI Webinar Series Pharma Trend Outlook 2022
As we approach the end of a year which saw global vaccine rollout for COVID-19, 44 novel drug approvals (to date) and the return of the CPHI Worldwide in-person event, we’re looking ahead to what 2022 has in store for the pharma industry. In this 60 minute, free-to-attend webinar, you’ll have the opportunity to hear from market experts from different sectors of the industry on the key trends, dynamics and disruptors we can anticipate in the coming year. Sourcing dynamics, the shift to self-administration, biotech innovation and sustainability are all high on the agenda – join us as we explore where the biggest opportunities and challenges lie and build your knowledge on where the industry is heading. -
News Recipharm signs agreement with Arcturus Therapeutics to support the manufacture of LUNAR[®]-COV19 (ARCT-021) vaccine candidate
Recipharm, a leading contract development and manufacturing organisation (CDMO) has entered into an agreement with Arcturus Therapeutics, a leading U.S. based clinical-stage messenger RNA medicines company focused on the development of infectious disease vaccines and significant opportunities within liver and respiratory rare diseases. -
Datasheet Combination inhalation devices
We have contributed to the design, development, and commercialisation of some of the world’s leading inhalation devices. -
News BIAL and Recipharm expand long-term supply agreement for opicapone API
Portuguese pharma company, BIAL, and global contract development and manufacturing organisation (CDMO), Recipharm, have expanded a long-term agreement for the global manufacturing and supply of BIAL’s proprietary molecule opicapone. -
Video Aseptic Manufacturing – Design of Lines for Filling and Lyophilisation
This Learning Lab session was originally broadcast as part of the CPHI Worldwide Content programme, this session will explore: Design and realisation of lines for filling and lyophilisation of drug products Development of a lyophilisation cycle for an mRNA vaccine candidate in 2 months Reduction of lyophilisation cycle during optimisation process -
News Sobrera Pharma and Recipharm in collaboration to advance a new treatment for patients with Alcohol Use Disorder
Swedish life science company, Sobrera Pharma, and global contract development and manufacturing organisation (CDMO), Recipharm, have entered into an agreement for the formulation development and manufacturing of SO-001, a new oral treatment for Alcohol Use Disorder (AUD). -
Video Recent Trends in Self-Administration Drug Delivery Systems
This session was originally broadcast as part of the CPHI Worldwide 2021 content programme. When we look at the evolution of the patient experience in drug delivery, we see that patients have traditionally been tethered to healthcare professionals, typically needing to visit a clinic to administer their treatment. With the arrival of new technologies, higher patient empowerment and better drug delivery mechanism, patients are starting to look for safe and more convenient manners moving toward self-administered rather than hospital-administered formulations. This session will explore: Growth, Competition, and Improved Access to Key Therapies Paving the Way for Increasing Advances in the Self-Administration Drug-Delivery Industry New Large-Volume Administration Devices Patient-Centricity Taking a Pivotal Role -
News Introducing Bespak by Recipharm
In February 2020, Recipharm acquired Consort Medical plc, which included Bespak. The move was designed to strengthen Recipharm’s position as a leading inhalation company and one of the largest CDMOs in the world. -
Video Accelerating Analytical Method Development using Non-Conventional Detection Techniques
This Learning Lab session was originally broadcast as part of the CPHI Worldwide 2021 digital content programme and will discuss: The challenging issues involved in analytical development using conventional detectors It will provide you with a broader discussion on non-conventional detectors and benefits from their usage The session will show some case studies involved in the development of methods using non-conventional detectors -
News Recipharm increases API and raw material stocks to ensure supply amid higher antibiotics demand
CDMO says it has made "notable changes" to its supply chain processes to ensure continuing supply of medicines during COVID-19 pandemic -
Brochure Self-administered injectable drugs: choosing the right delivery device for your product
In this eBook, we will explore how drug developers can best identify and develop the right drug delivery device for their self-administered injectable products. We will also highlight the potential disadvantages of poorly designed devices. We will provide practical, straightforward advice around choosing the right device for the needs of self-injected products, and the considerations that need to be made to ensure optimum performance and long-term patient acceptance as well as highlight the added value of engaging device experts early in the design and development process. -
Brochure Drug development for start-ups
While developing a new pharmaceutical product is one of the most interesting and rewarding activities to partake in, it is also extremely challenging. It is a given that drug development projects will not always succeed and the more innovative they are, the higher the likelihood of failure. There is no way to eliminate the risk of failure. However, there are ways to reduce these risks. -
Brochure Environmentally friendly propellants Open Environmentally friendly propellants configuration options
In recent years there has been a global focus on adopting sustainable processes, reducing carbon footprint and ultimately creating a ‘greener’ world for the future. The prominence placed on discussion around environmentally friendly operations has prompted widespread reviews of current practices across all industries, including the pharmaceutical industry. -
Brochure How to speed up vaccine development and production with genetic vaccine technology
We explore the challenges and pitfalls in developing new genetic vaccines, and in manufacturing them following approval. In addition, we discuss how strategic partnerships with expert contract development and manufacturing organisations (CDMOs) can support vaccine developers to bring their innovations to market.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































.png)
-file130276.png)
-file131321.jpg)




![Recipharm signs agreement with Arcturus Therapeutics to support the manufacture of LUNAR[®]-COV19 (ARCT-021) vaccine candidate](https://www.cphi-online.com/46/pdcnewsitem/08/51/99/p0th_S.jpg)










-page-001%20(1)-file121034.jpg)















-comp247874.png)











(1)v2.jpg)








.png)


















.png)









-comp245730.png)
.png)