Chemistry, Manufacturing and Controls (CMC)
Chemistry, Manufacturing and Controls (CMC) Companies (33)
Chemistry, Manufacturing and Controls (CMC) News
-
Sponsored Content CPHI Online Trend Report: How can flow chemistry help businesses achieve their sustainability goals?
In our latest CPHI Online Trend Report, we partner with Asymchem to understand the innovative potential of flow chemistry for API manufacturing, especially in regards to meeting sustainability goals. -
News Onyx Scientific expands small molecule CMC services with new UK facility
The contract development and manufacturing organisation (CDMO) has completed the first phase of its multi-million-pound development. -
News Inaugural Bio Integrates conference highlights industry's inefficiency in developing products
Industry leaders give voice to issues and trends shaping the biotech sector, including the importance of collaboration.
Chemistry, Manufacturing and Controls (CMC) Products (29)
-
Product Hi-End Resource On Demand Solutions
Project resource management includes forecasting the need to hire and/or outsource some tasks. When projects are high-value and require strategic decisions, in-house resources may be insufficient. Similarly, administrative action items are better left to contract workers so as to not overburden your staff....
-
Product in-vivo PK Studies
Our drug product pre-formulation and formulation development services can also includes in vivo PK studies, conducted with multiple animal species and a rapid turnaround time. This additional service further extends our full suite offering to our clients while eliminating the need for additional vendor sou...
-
Product Oligonucleotide Analytical Development Services
Analytical development services for oligonucleotides: For oligo-based drugs, our capabilities range from GLP bioanalysis to GLP/cGMP characterisation. We support product development, from quality control testing of amidite starting materials and early stage product characterization through to GMP batch r...
-
Product Cortellis CMC Intelligence™
Cortellis CMC Intelligence™ product is a comprehensive database of guidelines / granular collection of CMC data requirements for initial registration of small-molecule and biologics drugs around the world that can be used to avoid delays in product approval and successfully bring a drug to the market (laun...
-
Product A CDMO PARTNER FOR LIFE
FUJIFILM Diosynth Biotechnologies is an industry-leading cGMP Contract Development and Manufacturing Organization (CDMO) supporting the biopharmaceutical industry in the development and production of biologics, vaccines and cell and gene therapies. Our focus is to combine technical leadership in process d...
-
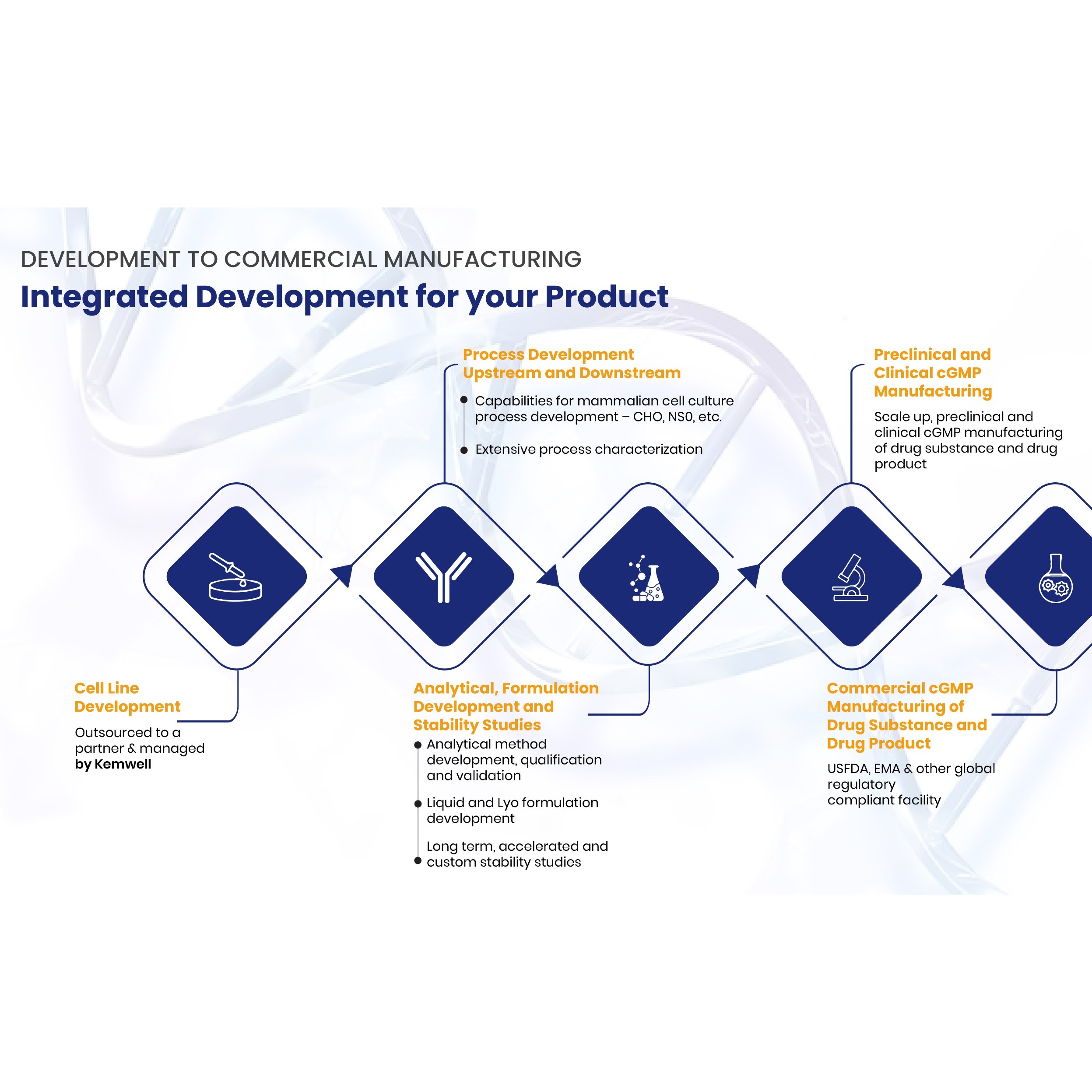
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product Gamma Irradiated Cleanroom Facemasks With Tyvek Side Extensions
Gamma Irradiated, Extra Large Cleanroom Facemask with Tyvek® Side Extensions, Green or Blue, with Double Elastic Headbands and with or without Fog-Prevention Adhesive are made from an inner facing of soft cellulose material, an outer facing of spunbonded polypropylene and a 100% meltblown polypropylene med...
-
Product Analytical Development
BioDuro-Sundia’s Analytical Testing team offers high quality analytical services including method development and validation, qualification of reference standards, testing and release studies, stability studies, and CMC dossier preparation services. ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product CDMO services - Spray-drying
Xedev can perform the clinical production for your spray-drying project.
-
Product CG-1978-B508
Refrigeration bath circulators are suitable for controlling the temperature of externally connected applications and thermoregulation of objects directly in the thermostat bath. Typical applications are: photometers, refractometers, viscometers, double-jacketed reaction vessels and autoclaves. The units ca...
-
Product Oral Dose Drug Product Development & Manufacturing
WuXI STA's comprehensive drug product development platform for oral dosage forms offers our clients a complete line of solutions from pre-formulation development through to final product manufacture at commercial scale. Our capabilities include;
• Solid oral tablet/capsule (including liquid...
-
Product Quality & Compliance Solutions
From early-stage development through commercial support, our experts provide critical GxP-based consulting services. We partner with clients to successfully execute projects throughout the product lifecycle to maintain quality & compliance with applicable regulations and industry standards. qq...
-
Product Regulatory Sciences
From early-stage development to post-approval, we partner with pharmaceutical, biotechnology, and medical device clients to overcome regulatory hurdles. Using science as the driver for success, we help our clients achieve positive regulatory outcomes with the Food and Drug Administration (FDA), Europea...
-
Product Nasal Drug Development
Development of nasal drugs: Scientists from our nasal drug development team provide method development, validation, and testing services to help you optimise the performance of your aqueous, powder, and propellant-driven nasal drug products. Conducted in Good Manufacturing Compliant (GMP) laborato...
-
Product GMP and CMC Laboratory Services
Laboratory services according to GMP and CMC: We provide regulatory-driven, phase-appropriate laboratory services, supporting CMC programs from preformulation to formulation to product release. Among our capabilities are centres of excellence for method development and validation, analysis, stability s...
-
Product Cortellis CMC Intelligence™
Cortellis CMC Intelligence product is a comprehensive database of guidelines / granular collection of CMC data requirements for initial registration of small-molecule and biologics drugs around the world that can be used to avoid delays in product approval and successfully bring a drug to the market (launc...
-
Product UV Light Blocking Tyvek Equipment Covers
UV Light Blocking Tyvek Equipment Covers are designed to keep cleaned pharmaceutical equipment and supplies clean and protected from external sources of contamination while also providing in-process protection to light sensitive biotech products.
-
Product Formulation Development
BioDuro-Sundia holds more than 25 years of experience formulating poor solubility and poor permeability drugs for the clinical studies. Our comprehensive suite of advanced formulation technologies and extensive scientific know-how supports >95% of marketed dosage form...
Upcoming Events
-
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



































































.png)







.png)

.png)



.jpg)


