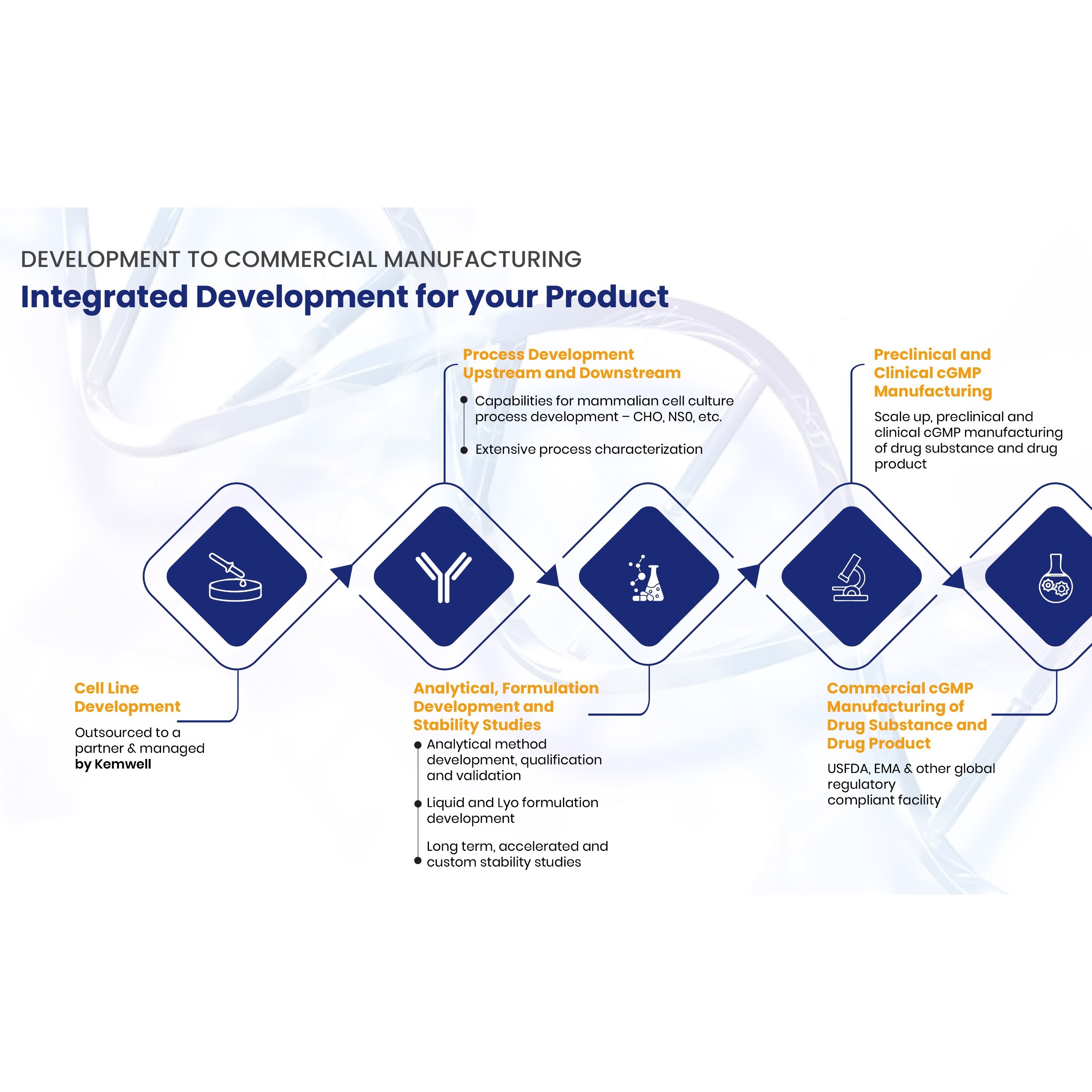
New Drug & Chemical Services

Product Description
Laviana Pharma Co., Ltd.

-
CN
-
2022On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Laviana Pharma Co., Ltd.

-
CN
-
2022On CPHI since
-
1Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Laviana Pharma Co., Ltd. (1)
-
Product Analytical and Quality Study Service
1.Quality Study on Drug Substance and Drug Product 2.Impurity Analysis Service 3.Stability Study 4.Analysis Service
Laviana Pharma Co., Ltd. resources (1)
-
News Laviana Pharma on the "TOP 10 Most Growing CXO Companies" List
The 6th China Bio-Pharm Partnering Forum was held in Suzhou from 4th - 5th August 2022. With its outstanding performance in multiple fields, such as innovative R&D, commercialization, and corporate growth, Laviana Pharma was selected as one of the “China Bio-Pharm Industry Value List 2022 - Top 10 Most Growing CXO Companies”.
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance