- Home
- GENEXT GENOMICS PVT LTD
- Biosimilar Clone Development Service
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Biosimilar Clone Development Service
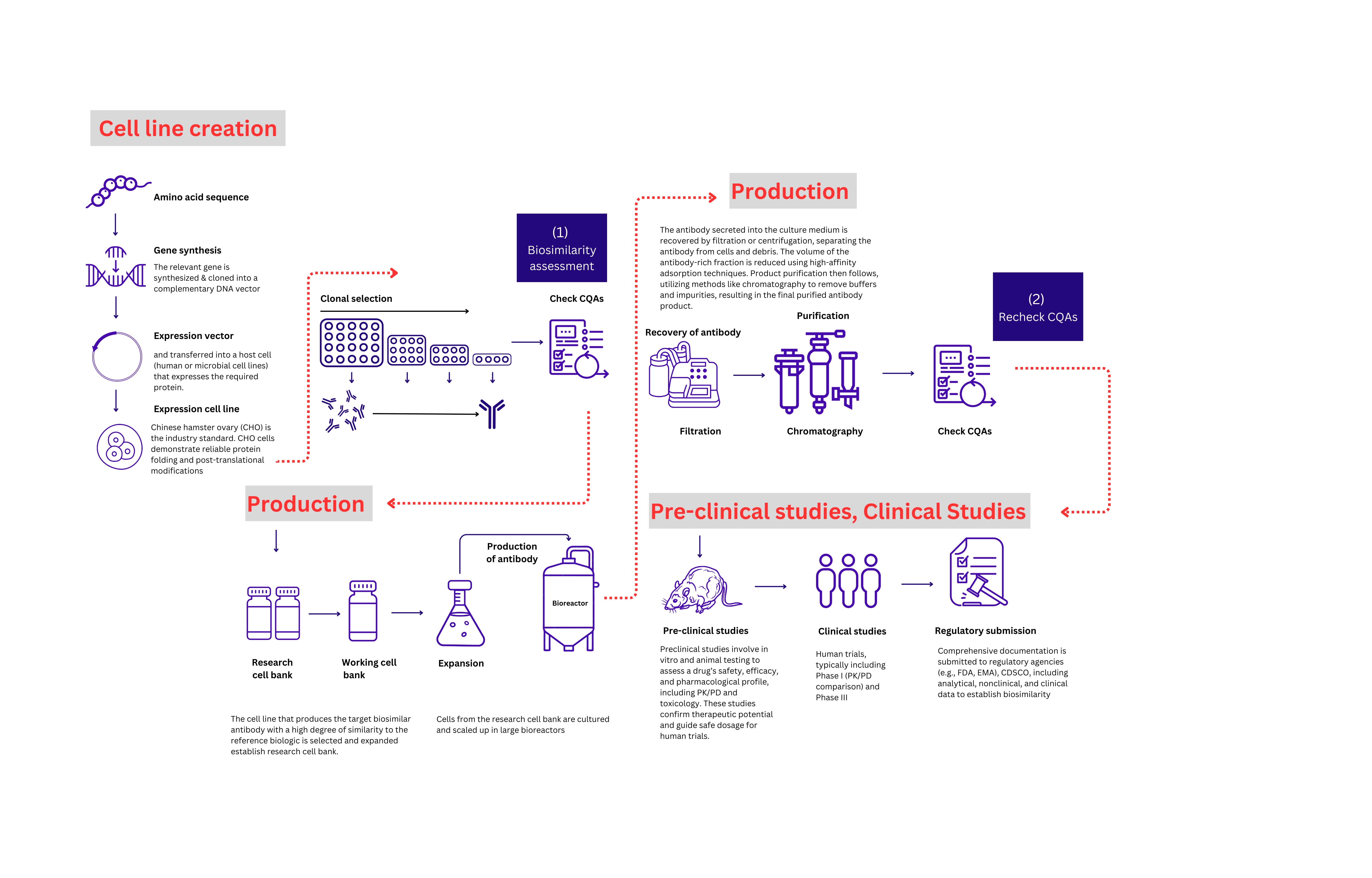
Product Description
At GeNext Genomics, we specialize in end-to-end clone development for biosimilars, catering to the high demand for biologics. Utilizing advanced cell lines such as CHO GS, Expi CHO, HEK, and CHO S, we ensure high-yield, high-quality biosimilar production. Our approach includes detailed characterization and strict alignment with CQAs from innovator molecules to meet regulatory standards. Through a robust, scalable process—from gene synthesis to cell line development and analytical validation—we support our partners in achieving cost-effective, market-ready biosimilars.
GENEXT GENOMICS PVT LTD

-
IN
-
2024On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Biopharmaceutical company
Contract Research Organisation (CRO)
Generics/Biosimilars Manufacturer
Primary activities
Analytical Services
Biopharmaceutical
Contract Research Organisation
Custom Manufacturing/Custom Synthesis
Categories
Specifications
- DetailsAt GeNext Genomics, we specialize in end-to-end clone development for biosimilars, catering to the high demand for biologics. Utilizing advanced cell lines such as CHO GS, Expi CHO, HEK, and CHO S, we ensure high-yield, high-quality biosimilar production. Our approach includes detailed characterization and strict alignment with CQAs from innovator molecules to meet regulatory standards. Through a robust, scalable process—from gene synthesis to cell line development and analytical validation—we support our partners in achieving cost-effective, market-ready biosimilars.
- Supplied fromIndia
- Measured Inmilligram
GENEXT GENOMICS PVT LTD

-
IN
-
2024On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Biopharmaceutical company
Contract Research Organisation (CRO)
Generics/Biosimilars Manufacturer
Primary activities
Analytical Services
Biopharmaceutical
Contract Research Organisation
Custom Manufacturing/Custom Synthesis
More Products from GENEXT GENOMICS PVT LTD (1)
-
Product Golimumab
Golimumab is a monoclonal antibody that targets and inhibits tumor necrosis factor-alpha (TNF-α), a cytokine involved in inflammatory responses. By blocking TNF-α, golimumab helps reduce inflammation and is primarily used to treat autoimmune diseases such as:
• Rheumatoid arthritis (RA) • Psor...
Recommended Products
-
Product Post-translational modifications
Intertek offers wide range of pharmaceutical services which includes post-translational modifications. It belongs to biopharmaceutical protein analysis services category. It includes deamidation, glycosylation, glycylation, phosphorylation, acetylation , alkylation, sulfation, etc.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product UV Light Blocking Tyvek Equipment Covers
UV Light Blocking Tyvek Equipment Covers are designed to keep cleaned pharmaceutical equipment and supplies clean and protected from external sources of contamination while also providing in-process protection to light sensitive biotech products.
-
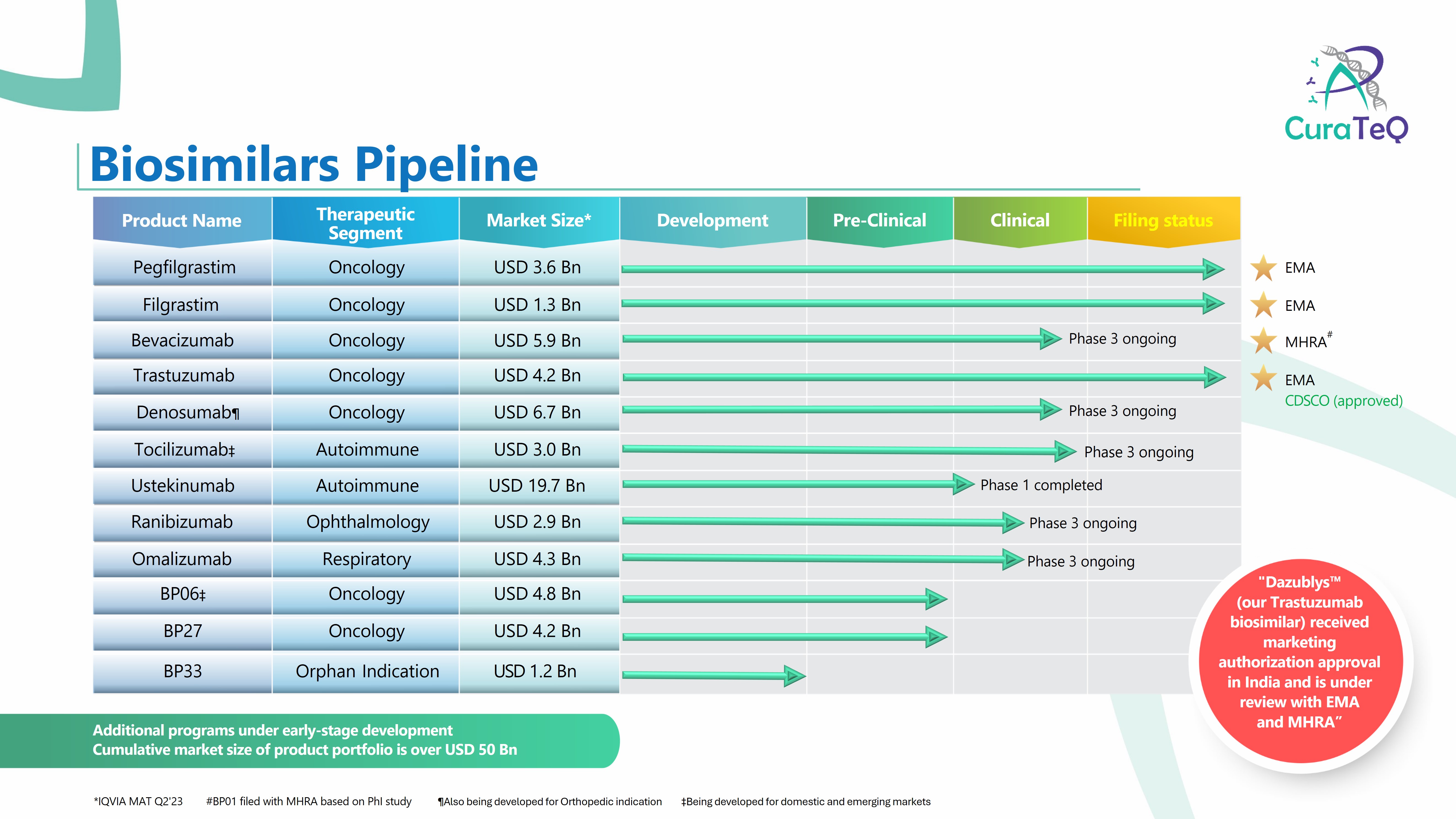
Product Biosimilars Portfolio
• Pegfilgrastim (Filed with EMA) • Filgrastim (Filed with EMA) • Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India) • Bevacizumab (Filed with MHRA) • Omalizumab (Global PhIII ongoing) • Denosumab (Global PhIII ongoing) • Ranibizumab (Global PhIII ongoing...
-
Product Biomedtracker
Biomedtracker provides real-time analysis of major market-moving events in the pharma and biotech industries. With catalyst tracking and expert insights, Biomedtracker helps you stay on top of breaking events, the drugs pipeline, upcoming milestones, companies, and deals – and their impact on the marke...
-
Product RoSS® Shell: Protection of single-use bags
Protect your single-use bioprocess containers: RoSS® Shells - abbreviated for Robust Storage and Shipping - reduce product loss towards 0%. Compatible with all available sizes and types of single-use bags, RoSS enables standardized and scalable end-to-end process solutions for fluid management and col...
-
Product INJECTABLES
Requiring injectables.EU-GMP is a must.
EU dossier is a must.
Licensing and supply model or distributorship.
-
Product Custom Protein Synthesis
We provide a Custom Protein Synthesis Service, using a chemical method that synthesises proteins amino acid by amino acid and making modifications on an atomic-scale. We work closely with our partners in designing, customising and optimising the proteins that is synthesized in an automa...
-
Product Recombinant human erythropoietin injection (cho cell)
Shandong kexing bioproducts offers a wide range of products which includes recombinant human erythropoietin injection (cho cell). It is colorless and transparent liquid with ph6.9±0.5. Packaging: pre-filled syringe or vial. Storage: stored at 2-8?. Contact us for more information.

-
Product Monomaterial Packaging Specialist
Pharma and Biotech toploading and sideloading cartoning systems from the monomaterial specialistDividella from Switzerland provides pharma and biotech packaging systems for the secondary packaging of parenterals. Dividella's patented top-loading and recently also side-loading cartoners package syringes, vi...
-
Product FPFs
• Genexol®PM Inj.(Paclitaxel) • Genexol® Inj.(Paclitaxel) • Nanoxel®M Inj.(Docetaxel) • Docetaxel Inj.(Docetaxel) • Nexatin® Inj.(Oxaliplatin) • Everose® Tab.(Everolimus) • Pemed®S Inj.(Pemetrexed) • Azalid® Inj.(Azacitidine) • Decilid® Inj.(Decitabine) • Lenalid® Inj.(Lenalid...
-
Product Romiplostim Biosimilar Candidate
Due to the complex nature of the product, Paras Biopharmaceuticals has been intensively working on this complex drug (Nplate™) for over 6 years and has progressively achieved success. The company plans to mature good value within its Romiplostim asset (Biosimilar) and would like to highlight the following...
-
Product HANBEITAI (Bevacizumab Injection)
HANBEITAI (generic name: bevacizumab), the fourth product independently developed by Henlius, received marketing approval from the National Medical Products Administration (NMPA) in November 2021, and its commercialization in Chinese mainland is handled by the company's in-house team. As of now, HANBEI...
-
Product Sterile Grade at ISO Class 5 facility
Kikkoman can offer "sterile grade” products, purified through a super purification process at our ISO Class 5 facility, approved by the Japanese Authority.
-
Product Biotechnology Services
Midas Pharma offers a wide range of pharmaceutical services which include biotechnology services. It offers: • development of biosimilars and proprietary biologicals (starting from gene sequence) • cGMP production (microbial and mammalian cells) • related bioanalytics (from protein biochemical meth...
-
Product Ready-to Use CHO cell lines
UGA Biopharma GmbH offers to their clients so called Ready-to-use Biosimilar Cell Lines. This are in-house developed CHO cell lines stably expressing different biosimilar molecules, that are instantly available for in-licensing. The client profits, because the cell lines are directly available, and develop...
-
Product Onko BCG 100
BCG for immunotherapy.
The product is intended for treatment of superficial, epithelial non-invasive urothelial carcinomas (carcinoma urotheliale Ta, Tis, T1)
1 ampoule or 1 vial with the powder contains:
Live attenuated bacilli Bacillus Calmette-Guerin, Brazilian BCG Moreau substrain ...
-
Product DWRX1010 (Colonoscopy Bowel Preparation Drug)
※ DWRXs Differentiation Points 1) Maximize compliance in the form of minitablets. 2) Containing simethicone to remove foam in the colon, enabling smooth colonoscopy. 3) Maximizes the convenience of taking medication, and low water intake. ※Development Strategy 1) Non-clinical trial (KR) and patent applica...
-
Product N-Acetyl-DL-Tryptophan (Ph. Eur, BP) low endotoxin, pharma grade CODE 63...
https://www.itwreagents.com/iberia/en/product/n-acetyl-dl-tryptophan-ph-eur-bp-low-endotoxin-pharma-grade/637763
-
Product Yuflyma
• Ingredient: Adalimumab • Indication: Rheumatoid arthritis, inflammatory bowel disease • Mode of mechanism: Blocks the transmission of inflammatory signals by binding to TNF-α in the body • Approval status: 44 countries including the US(product name: Inflectra), Europe and South Korea-comp304625.png)
-
Product Basalin®(insulin glargine injection)
Basalin® is an insulin glargine injection developed and produced independently by Gan & Lee Pharmaceuticals. It was launched in China in 2005 and has been approved in almost 20 countries globally. As a long-acting biosimilar insulin, Basalin® is able to stablely control human blood sugar within 24...
-
Product Hoses and Hygenic Connections
Our hoses for pharma biotech, cosmetics, food, beverage and chemical industry
We offer you a wide range of hoses specially designed for the pharmaceutical, biotech, cosmetic, food, beverage and chemical industries. We also ensure their proper use through training in the co...-comp318375.png)
-
Product Heparin-Indar
Prjsc "Indar" provides wide range of antithrombotic products which includes heparin-indar. The product of direct anticoagulant group. Heparin acts fast. Is applied for treatment of thrombembolia and diseases that have risk of thromboembolic complications, for prevention of post surgery venous thrombosis an...
-
Product Sodium Hyaluronate - Injection grade
Hyature® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyature® sodium hyalur...
-
Product Fluid Transfer
A full range of tubing and hoses best suited for the biopharma and pharmaceutical markets. From TPE to silicone, each material type offers a unique set of features and benefits. ValPlus™, an enhanced level of tubing validation certification, is available for select formulations.
-
Product LYCADEX® BioPharma - Dextrose monohydrate, low endotoxin, USP, EP, JP
Delivering secure supply with the right partner. Trust is the most essential ingredient.
-
Product Eylea biosimilar vial & PFS
1.Completion of global clinical trials phase Ⅲ
2. Completion of pre-submission meetings with advanced regulatory authorities
3. Available in Vial and PFS
-
Product Sterility Test Isolator
1. Built-in Vaporized Hydrogen Peroxide (VHPS) generator for bio-decontamination which can reach 6-log Sterility Assurance Level
2. The VHPS residual concentration is less than 1ppm after aeration
3. leakage rate is less than 0.5% /vol/hour under 2 times working pressure test
qq...
-
Product RARE DISEASES & ORPHAN DRUGS
Lodaat has a portfolio pipeline of Rare Disease and Orphan Drug products. USFDA in development, we are looking for partners for the US, GCC, LATAM, EU markets. Many products are made in USA products in FDA approved facilities.
-
Product Tofflon Integrated Solution for Biologicals MFG
Applications: Biosimilars& Therapeutical Protein Nucleic Acid-based Vaccine(mRNA/DNA) Recombinant Viral Vector Vaccine Inactivated/Attenuated Viral Vaccine Gene Therapy

-
Product Biologics
End-to-end development and manufacturing capabilities across different therapeutic modalities and the entire product lifecycle
Cutting-edge technology platforms for high yielding cell lines (both mammalian and microbial) and high concentration formulations
Integrated and flexible drug sub...
-
Product Interview by Newsweek, time to talk
Interviewee:Dr. Steve J.P. Hsu (President of Genovior Biotech)
-
Product Method Development
We are specialist in the method development of analytical methods for analysis of API, Final Drug Products or low level impurities. Usually followed by full validation in accordance to ICH guidelines.
-
Product Cortellis CMC Intelligence™
Cortellis CMC Intelligence™ product is a comprehensive database of guidelines / granular collection of CMC data requirements for initial registration of small-molecule and biologics drugs around the world that can be used to avoid delays in product approval and successfully bring a drug to the market (laun...
-
Product CannEpil
CannEpil®, one of Argent BioPharma's flagship products, is an enhanced iteration of a compounded isolated cannabinoid formulation. It leverages well-known historical data to become one of the first compounded prescription investigational drugs used for seizure management over the past years. The active ing... -
Product BioPharma
When you know the biopharma solution you need and want to find the perfect delivery partner, Caldic has the expertise to source, process and customize packaging to your exact requirements. From packaging and single use solutions to stainless steel vials, and following allergen, GMO or REACH regulatory requ...
-
Product Rituximab
Rituximab binds itself to the CD20 protein, marking them, and subsequently triggering the cells of the immune system to pick out the marked cells and kill them.The indication is for Non Hodgkin Lymphoma
-
Product Mipeginterferon alfa-2b
Pegylated human interferon alfa-2b
Trade name: PEGBING
Technology
Recombinant gene technology, expressed by Yeast
Amino acid
165 amino acids
Molecular weight
Approximately 59 kD
Indications
Chronic hepatitis B & C
...
-
Product Millistak+® HC Pro Synthetic Depth Filter
Millistak+® HC Pro (high-capacity synthetic media) is a family of synthetic depth filters providing a cleaner and more consistent depth filtration media over current diatomaceous earth (DE) and cellulose (CE) based filter offerings. Multiple media grades are available for primary and secondary clarificatio...
-
Product Biotech Development
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of biotech services such as strain (including MCB and WCB Production) and process development - including special experience in microbiom formulation - up to clinical phase III. Contact us for more information.
-
Product Icatibant
is a synthetic peptidomimetic drug consisting of ten amino acids, and acts as an effective and specific antagonist of bradykinin B2 receptors. It has been approved in the EU for use in hereditary angioedema, and is under investigation for a number of other conditions in which bradykinin is thought to play ...
-
Product Antide
CAS No. : 112568-12-4.Molecular Formula: C82H108ClN17O14 Application: Anti-microbial Main product: Oxytocin Acetate,Vasopressin Acetate,Desmopressin Acetate,Terlipressin acetate, Caspofungin acetate, Micafungin sodium, Eptifibatide acetate, Bivalirudin TFA, Deslorelin...
-
Product Patux solution
Patux solution thanks to its composition studied for a rapid and effective action, is useful in case of dry cough.
DOSAGEChildren above 2 years: 2,5 ml three times per day. Adults: 5 ml three times per day.
INGREDIENTSAlthea officinalis roots E.S., Plantago major L., Marrubi...
-
Product Chorionic Gonadotrophin (HCG)
DADELI is a GMP-certified pharmaceutical manufacturer for intermediates,APIs and finished formulations.Our main APIs include HCG, Aprotinin(Currently in China we are the only one to produce Aprotinin complying with the latest EP 11.0), Oxymatrine,Isoniazidum,Egg Lecithin,Daidzein,etc.Warmly welcome all cus... -
Product Eye drop Grade Sodium Hyaluronate (Hyaluronic Acid )
Eye-drop Grade Sodium Hyaluronate has GMP certification in China and the status of API registration status is A. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
• M...
-
Product Biopharmaceutical Analysis
Solvias has a solid reputation with market-leading biopharmaceutical companies for the characterization and QC analysis of monoclonal antibodies (mAbs), glycoproteins, PEGylated proteins and peptides and biosimilars (follow-on biologics).. This means that businesses of all sizes, even smaller biotechs, can...
-
Product WHEATON Roller Apparatus by DWK
WHEATON® roller apparatus are used for roller bottle cell culture, an established cell culture method and relatively inexpensive way to set up a flexible and scaleable biopharmaceutical operation. In addition to the standard product DWK also offer a wide range of customized options to accommodate the most ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






.jpg)