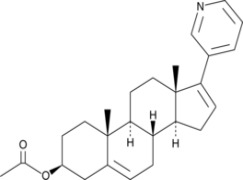
Apremilast

Product Description
Dr Reddys Laboratories Ltd

-
IN
-
2019On CPHI since
Specifications
Dr Reddys Laboratories Ltd

-
IN
-
2019On CPHI since
More Products from Dr Reddys Laboratories Ltd (5)
-
Product Abiraterone Acetate
We supply Abiraterone Acetate in bulk quantities. Learn more on api.drreddys.com. -
Product Atorvastatin
We supply Atorvastatin as a generic API in commercial quantities. -
Product Apremilast
As one of the largest API supplier worldwide, we are your reliable partner for the supply of high quality commercial quantities of Apremilast -
Product Apremilast
As one of the largest API supplier worldwide, we are your reliable partner for the supply of high quality commercial quantities of Apremilast -
Product Apremilast
As one of the largest API supplier worldwide, we are your reliable partner for the supply of high quality commercial quantities of Apremilast
Dr Reddys Laboratories Ltd resources (4)
-
News CPHI Barcelona 2023: Tackling the Pharma Talent Precipice – Part 1
This year at CPHI Barcelona (24–26 October, 2023) we sat down with C-suite executives and HR professionals to discuss the looming talent crisis in the pharmaceutical industry. With hybrid working persisting post-pandemic and a growing skills gap, how can the pharmaceutical supply chain adjust to a changing labour force? -
News Novartis agrees for copies to be made of cancer drug to reach poorer countries
Novartis signs agreement with MPP to have generics of it's leukemia drug made so that it can be more easily distributed to the world's poorer countries. -
News Aurigene Pharmaceutical Services to expand biologics CDMO capacity with a new biomanufacturing facility
Aurigene Pharma announces the construction of a state-of-the art development and manufacturing facility for therapeutic proteins, antibodies, and viral vectors.
As a first step of investment plan, Aurigene is investing $40m in an R&D and pilot scale facility at Genome Valley, a well-known Biotechnology Park in Hyderabad. This facility will meet the process development and clinical supply needs of global biotech companies. The facility is planned to be fully operational in first half of 2024.This complements Aurigene’ s strong foundation in biotherapeutics discovery, contributing to accelerating global R&D-driven (bio)pharma companies’ biologics journey to the market by offering end-to-end and high-quality services for antibodies, proteins, and viral vectors. -
News CPHI Podcast Series: The importance of Pharma 4.0 to innovation leads in India
In this latest episode from the CPHI Podcast Series, Digital Editor Lucy Chard speaks to Sanjay Sharma, Global Head of Manufacturing, from Dr Reddy’s. Sanjay has over 26 years of experience in roles encompassing sales, the supply chain, and technical operations.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



.png)


