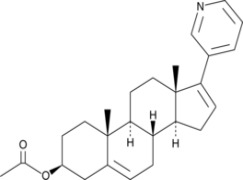
Atorvastatin

Product Description
Dr Reddys Laboratories Ltd

-
IN
-
2019On CPHI since
Specifications
Dr Reddys Laboratories Ltd

-
IN
-
2019On CPHI since
More Products from Dr Reddys Laboratories Ltd (2)
-
Product Abiraterone Acetate
We supply Abiraterone Acetate in bulk quantities. Learn more on api.drreddys.com. -
Product Apremilast
As one of the largest API supplier worldwide, we are your reliable partner for the supply of high quality commercial quantities of Apremilast
Dr Reddys Laboratories Ltd resources (2)
-
News CPHI Barcelona 2023: Tackling the Pharma Talent Precipice – Part 1
This year at CPHI Barcelona (24–26 October, 2023) we sat down with C-suite executives and HR professionals to discuss the looming talent crisis in the pharmaceutical industry. With hybrid working persisting post-pandemic and a growing skills gap, how can the pharmaceutical supply chain adjust to a changing labour force? -
News Novartis agrees for copies to be made of cancer drug to reach poorer countries
Novartis signs agreement with MPP to have generics of it's leukemia drug made so that it can be more easily distributed to the world's poorer countries.
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


.png)
