6100A / 6180A Paperless Graphic Recorder

Product Description
Watlow

-
US
-
2023On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Watlow

-
US
-
2023On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Watlow (9)
-
Product FLUENT® In-Line Heaters
Watlow’s new FLUENT® in-line heater is a small, lightweight, high-performance heater that can replace both a traditional immersion type heater or a heater wrapped around a tube as part of a thermal system. FLUENT features an all stainless steel construction for a clean, non-contaminating heat source. ... -
Product Digital Engineered Solutions for Environmental Monitoring Systems
Eurotherm DES for EMS are designed to aid compliance to FDA 21 CFR Part 11, EudraLex Annex 11, and Good Practice guidelines. From an entry-level architecture based on Eurotherm self-contained recorders, to a high availability architecture including redundant instrumentation, virtualized servers, cybersec... -
Product Eurotherm Data Reviewer
Eurotherm Data Reviewer is a software application designed for the viewing, analysis and printing of historical data files obtained from Eurotherm data acquisition equipment.
Find and analyze data quickly, including by instrument group or batch.
The Audi... -
Product Polyimide Flexible Heaters
Solvent-resistant polyimide is a thin, lightweight organic polymer film that provides excellent tensile strength, tear resistance and dimensional stability. Watlow’s polyimide heater is ideal for IV heaters, respiratory therapy units and blood, fluid and endoscope warmers. It is also used for DNA analy... -
Product nanodac™ Recorder Controller
A compact 1/4 DIN recorder with precision PID control option.
• Digital batch recording and electronic signatures aid compliance to FDA 21 CFR Part 11 regulation and Data Integrity ALCOA+ principles • Can reduce capex using preset ISPE GAMP® 5 validation document templates and built in f... -
Product ULTRAMIC® Advanced Ceramic Heaters
ULTRAMIC® advanced ceramic heaters are designed for medical devices and clinical applications where electrical safety, small size, fast temperature ramp rates and cleanliness are critical. Applications such as respiratory therapy equipment, clinical diagnostic instrumentation and DNA analysis are an ideal ... -
Product Syringe Heaters
Watlow syringe heaters were developed specifically to match the unique needs of medical injection applications. Fluid and drug delivery that maintains precise liquid temperatures and reduces fluid viscosity maximizes patient comfort and decreases risk. Body temperature injections are more easily introd... -
Product T2750 Programmable Automation Controller
Provides high-performance control and recording with high availability options in a versatile modular format. • Offers Distributed Control System (DCS) performance • Analog, logic and sequential control with alarm management • High precision control/high accuracy measurement • Cost effective redun... -
Product FIREROD® Cartridge Heaters
FIREROD® cartridge heaters are designed for medical equipment including baby incubators and kidney dialysis machines and injector ports. Incorporating engineering excellence and supported by more than half a century of solid industry performance, Watlow’s FIREROD cartridge heater is an excellent soluti...
Watlow resources (6)
-
Video Watlow vision for Life Sciences. We start with compliance.
Focused on key applications and following Quality by Design (QbD) principles, we continue to invest in the development of digital engineered solutions (DES) to aid GMP, regulatory compliance and efficiency optimization and help reduce capital project execution costs, risks and time to market, as well as offering services that help lower operational expenditure. -
Whitepaper How to Meet Data Integrity ALCOA+ Principles
Proof that pharmaceutical ingredients and products have been made correctly and are safe to use is reliant on trustable data from the manufacturing process and its supply chain. The Data Integrity ALCOA+ concept defines best practice guidelines and methodologies for good data management within life science industries. This paper gives an overview of the ALCOA+ concept, its role within the digital transformation of the Life Science Industry, and offers a view on data acquisition and management solutions that help achieve the required data integrity -
Whitepaper Life Sciences Automated Environmental Monitoring Systems: Regulations and Guides
This document is a compendium of key European and North American rules and regulations governing environmental monitoring of storage and production in the Life Sciences industry -
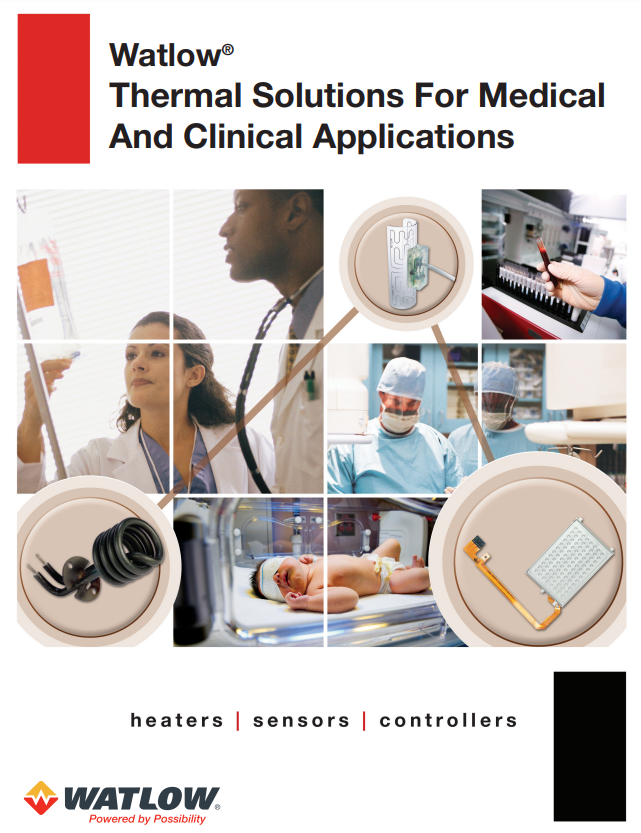
Brochure Watlow® Thermal Solutions for Medical and Clinical Applications
Temperature continues to play an important role in the development of new medical equipment, biotechnology and clinical diagnostic instrumentation. With 50 years’ experience in the Life Science industries, Watlow® is a leader in breaking new ground in designing, manufacturing and supporting innovative thermal solutions to meet medical advancements and new industry requirements. Leading medical equipment manufacturers rely on Watlow solutions for their patientcare equipment, surgical devices and biotechnology and clinical diagnostic instrument application needs.
Greater temperature responsivenessAccurate temperature control of critical processesLow leakage current complianceReduced cost, space and device weightSuperior temperature measurementLower heater mass and temperature uniformityFaster processing timesReduction in moving partsProduct miniaturization -
Brochure Efficiency Solutions for the Life Science Industry
Eurotherm provide a range of products, engineered solutions and services throughout the world. We can help to solve your manufacturing challenges:Development cost and time to market (data analytics and knowledge management)Constraints linked compliance (Quality by Design)Improving process efficiency (through quality and cost control)Adapting to patient centred care (personalised medicines)Target carbon neutrality -
Video Digitalize Environmental Monitoring aiding regulatory compliance
ISPE guidance suggests considering the segregation of the Building management system (BMS) and the Environmental monitoring system (EMS). Segregating the control system (BMS) from the monitoring system (EMS) enables significant savings, in fact: qualification documentation and execution challenges apply to EMS only, change control and risk assessment activities are not required for modification to the BMS, periodic calibration routines are limited to the EMS, record management is limited to the EMS critical parameter data and its contextual metadata.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance