20
May
2025

Adare Pharma Solutions
Exhibitor at CPHI Americas 2025 stand 1312 & stand 330
About Us
Categories

-
US
-
2015On CPHI since
-
2Certificates
-
500 - 999Employees
Company types
Primary activities
Event information
CPHI Americas 2025
-
20 May 2025 - 22 May 2025
-
Pennsylvania Convention Center, Philadelphia
-
Visit us at stand 1312
Products Featured at CPHI Americas 2025
-
Product Manufacturing Capabilities
Standard Offerings
• Granulation & Mixing • Fluid Bed Processing & Drying • Hot Melt Extrusion • Pan Coating • Blending • Tableting • Multi-layer Tablets • Capsule Filling • Oven Drying • Small-scale GMP Manufacturing ... -
Product Development Services
Comprehensive R&D Services • Preformulation • Formulation Development of Solid Dose Tablets (Capsules, Liquids & Solutions) • Pediatric Formulation • Product Design • Formulation Optimization • Sourcing of APIs & Excipients • Analytical Method Development & Validation • ... -
Product Technologies
Adare’s industry-leading experts possess unparalleled experience in the development of unique dosage forms that provide taste masking, customized release, and patient-centric solutions. Our technology platforms overcome complex formulation challenges to improve the lives of all patients, with expertise in ... -
Product Packaging
Clinical & Commercial Packaging Services
• Two FDA-Registered Packaging Facilities • A Full Range of Solid-Dose Packaging Solutions • High-Speed Bottle Filling • Blister Packaging • Stick Pack & Cartoning Operations • Packaging of DEA Schedules II, 2N, III, 3N, IV, L1 • Thermo &... -
Product Nitrosamine Mitigation
Very few CDMOs can claim Adare's level of experience in mitigating the presence of nitrosamine. We can employ our own-in-house mitigation processes to develop the best long-term control strategies for your product.
Regain control of your product with NitroCLEARx • Receive real-world mitiga... -
Product Adaptdose™
Adaptdose™, Adare’s unique commercialized approach to adaptive formulation design, is a highly flexible, multi-delivery platform tool that eliminates the need for post Phase 1 trial reformulations and facilitates the creation of custom oral delivery systems. Adaptdose provides the ability to combine 2-3 ac... -
Product CAT.one SM
CAT.one SM minimizes the potential for abuse by ensuring that drug products are evaluated for all potential routes of misuse. Adare’s experts integrate abuse-deterrent testing by replicating basic and multi-step methods that an abuser may use to prepare the product for recreational misuse or abuse. Our CAT... -
Product DIFFUTAB® Technology
DIFFUTAB® technology is an effective solution for targeted drug delivery through customized and sustained release. The technology assists the development of high-dosage and sustained release products for once-daily administration. A matrix tablet is coated with functional polymers, followed by a blend of h... -
Product Duragran™
Duragran™ allows for more uniformity and precise control of targeted particle sizes while providing production time and cost savings. It eliminates the need for extrusion and spheronization for high API-loaded drug particulates by using fluid bed processing technology that combines the particle sizing and ... -
Product Microcaps® Taste Masking and Modified Release Technology
Microcaps® Taste Masking and Modified Release Technology achieves uniform and efficient coating of drug particles by coacervation (phase separation) to build polymeric membranes of varying porosity and thickness. The result is a free-flowing powder containing microencapsulated API (or API substrate) in a w... -
Product Precision Particle Fabrication™ Technology
Precision Particle Fabrication™ technology produces uniform microspheres and microcapsules with narrow size distribution and precise control over particle structure. The platform technology is flexible and customizable to accommodate a broad range of active ingredients, including small molecules, peptides ... -
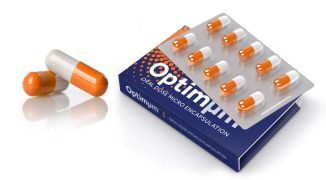
Product Optimµm® Technology for Oral Delivery
The Optimµm® technology creates microspheres with high drug loading suitable for taste masking, enteric coatings, and extended release oral applications. The technology is capable of producing single- or double-layer particles in a one manufacturing step, lending to cost-effective creation of palatabl... -
Product Stratµm™ Technology for Injectable Delivery
The Stratµm™ technology, for injectable delivery, allows for precise control of particle size and composition enabling predictable injectable performance, which is critical for self-administration, extended release, and modified release applications with a narrow therapeutic index. Unlike other encapsu... -
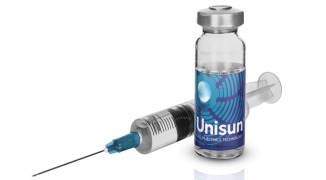
Product Unisun® Technology for Otic Delivery
The Unisun® technology combines the use of encapsulated or free drug, along with a fast-film forming agent, which allows for low-cost, long-acting intratympanic delivery of a therapeutic. The technology allows for less frequent administrations than current therapies and is capable of sustaining drug le...
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance