28
Oct
2025

IDT Biologika GmbH
Exhibitor at CPHI Frankfurt 2025 stand 6.0C54, Contract Manufacturing and Services
About Us
Categories

-
DE
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Event information
CPHI Frankfurt 2025
-
28 Oct 2025 - 30 Oct 2025
-
Messe, Frankfurt
-
Visit us at stand 6.0C54, Contract Manufacturing and Services
Products Featured at CPHI Frankfurt 2025
-
Product Drug Substance Manufacturing for Viral Vaccines, Gene & Immune Therapeutics, Oncolytic Viruses and Viral Vectors
Drug Substance Manufacturing for pre-clinical, clinical and commercial supply- Validation of aseptic processes - Cell and virus banking - Using own seed cell banks: VERO and DF-1, Duck cell line (AGE-1) - API production using different TechnologiesMVA Know-How IDT Biologika is considered the global lea... -
Product IDT Biologika as a Contract Development and Manufacturing Organization
IDT Biologika is an international leader in the contract development and manufacturing of biologics. The company focuses on the customized development and manufacturing of viral vaccines (phases I, II, III), gene therapeutics, immunotherapeutics, oncolytic viruses as well as sterile liquid and lyophilized ... -
Product Packaging, Quality Control, Storage, Cold Chain
Within the contract development and manufacturing, IDT Biologika GmbH provides a wide range of other pharmaceutical services for our clients products, which include- Labeling & Packaging of vials, prefilled syringes, autoinjectors - Serializiation (Track & Trace) - Quality Systems - Cold Chain up to - 80- ... -
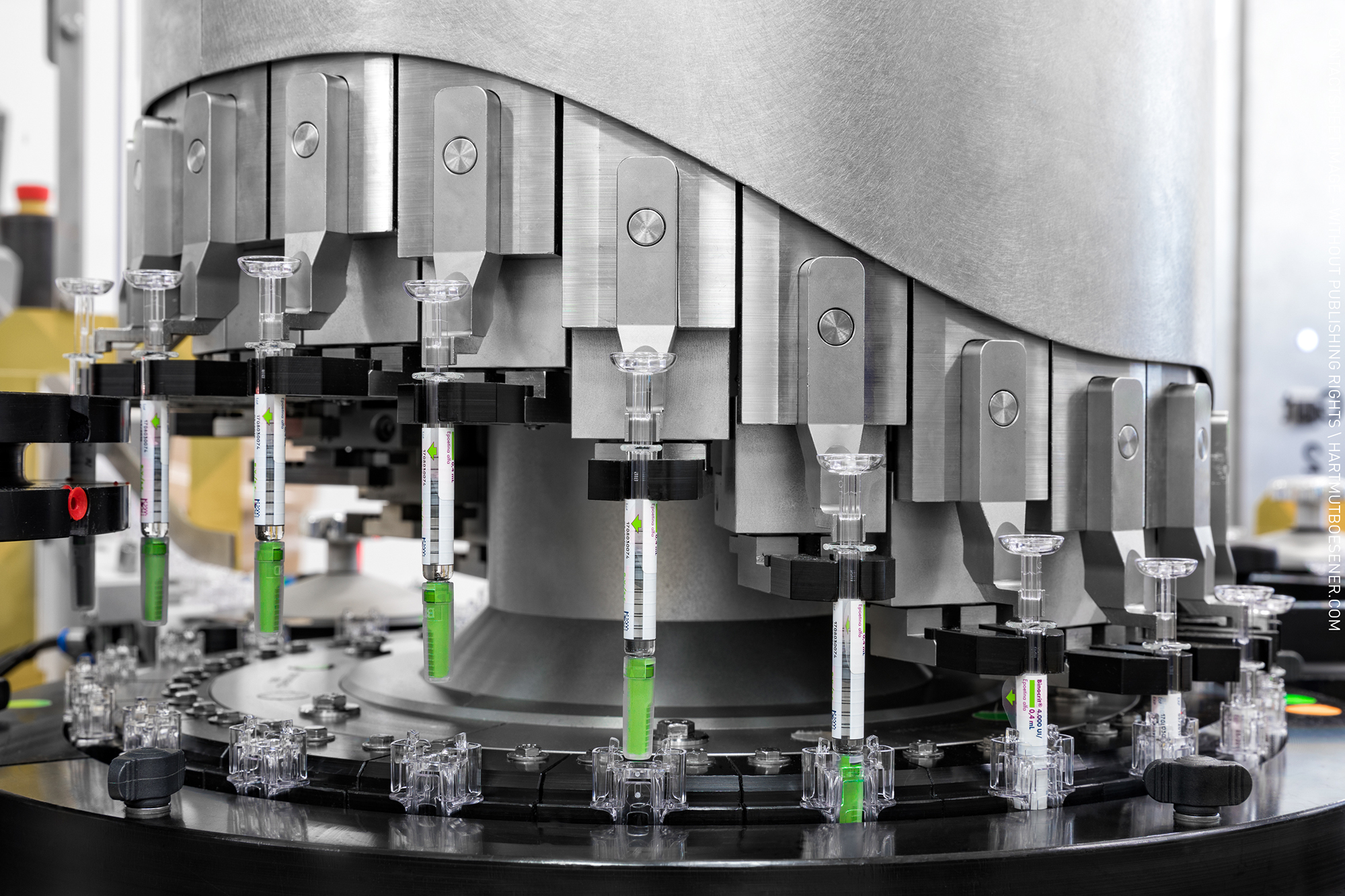
Product Sterile Dosage Filling and Lyophilization
IDT Biologika GmbH provides a wide range of pharmaceutical services which includes sterile dosage filling in vials and pre-filled syringes. With a facility size of 3,600m2, this integrated pharmaceutical/ biological site houses more than 7 filling lines for the aseptic filling of syringes, vials, ampoules ... -
Product Process Development for Production of Viral Vaccines and Vectors
Process Development for API Production, Fill/Finish and Secondary Packaging- Cell line development - Transfer of customer technologies -Upstream / Downstream -iCellis Fermenter: development of scalable upstream and downstream technologies for adherent cells with higher yield.Major advantage for devel...
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance