28
Oct
2025

Nordmark Pharma GmbH
Exhibitor at CPHI Frankfurt 2025 stand 6.0C89, Contract Manufacturing and Services
About Us
Categories

-
DE
-
2015On CPHI since
-
2Certificates
-
500 - 999Employees
Company types
Primary activities
Event information
CPHI Frankfurt 2025
-
28 Oct 2025 - 30 Oct 2025
-
Messe, Frankfurt
-
Visit us at stand 6.0C89, Contract Manufacturing and Services
Products Featured at CPHI Frankfurt 2025
-
Product Pancreatin powder
Nordmark Pharma GmbH provides a wide range of biological APIs which include pancreatin powder produced in Germany. It is differentiated by excellent free-flow properties and a high bulk density. The material can be used for direct compression tablet formulations. Diverse pancreatin powder product quali... -
Product Enteric-coated Pancreatin pellets
Nordmark Pharma GmbH provides a wide range of products based on our own produced biological APIs produced in Germany, which include enteric coated pancreatin pellets with high specific enzymatic activity and therefore patient-friendly capsule sizes. Diverse pellet product qualities according to custome... -
Product Film-coated Pancreatin micro tablets
Nordmark Pharma GmbH provides a wide range of drug products based on our own biological APIs produced in Germany, which include enteric-coated pancreatin micro tablets. Contrary to pellets, micro tablets are uniform in shape and the amount of active pharmaceutical ingredient of each tablet is exactly defin... -
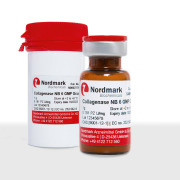
Product Collagenase NB
Apart from biological APIs Nordmark Pharma GmbH provides Collagenase NB derived from Clostridium histolyticum. Collagenase NB is a chromatographically purified mixture of enzymes used for tissue dissociation in-vitro and thus applicable for isolation of many different types of cells. The isolated cells ... -
Product Collagenase AF
Apart from biological APIs Nordmark Pharma GmbH provides Collagenase AF derived from Clostridium histolyticum using an animal free (AF) cultivation media. Collagenase AF is a chromatographically purified mixture of enzymes used for tissue dissociation in-vitro and thus applicable for isolation of many ... -
Product Collagenase N powder
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of biological APIs, including Collagenase N powder. Our collagenase is an enzyme preparation produced by fermentation using Clostridium histolyticum followed by purification and subsequent aseptic lyophilisation. At Nordmark, this asep... -
Product Process and formulation development
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of services such as process (fermentation and purification) and formulation development especially for biological drug substances/ products up to clinical phase III including special experience in microbiom formulation. Contact us for more i... -
Product Investigational Medicinal Product Manufacture
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of services, including the manufacture of investigational medicinal products (IMP), in particular based on biological APIs. Capability to produce microbiom IMP under anaerobic conditions. Contact us for more information. -
Product Biotech Development
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of biotech services such as strain (including MCB and WCB Production) and process development - including special experience in microbiom formulation - up to clinical phase III. Contact us for more information. -
Product Pankreatan® and Pankreatin Nordmark®
Nordmark Arzneimittel GmbH & Co. KG provides finished drug products for the treatment of the gastrointestinal system, produced in Germany from our own API and designated for GI-treatment. This includes the brand Pankreatan® and Pankreatin 40.000 Nordmark®, pankreatin containing enteric coated micro ... -
Product Thrombophob®
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of biological drug products made from our own APIs produced in Germany. This includes Thrombophob® 60.000, a heparin ointment with 2 alternative ointment bases: a) gel b) creamogel. Contact us for more information. -
Product Burlulipase
Burlulipase is a pure, liquid, animal- and virus-free, highly active, and acid-stable lipase-only product from bacterial fermentation. This enzyme is tested in clinical trials with patients suffering from the failure or impairment of the pancreatic digestive function (exocrine pancreatic insufficiency, ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance