Zoledronic Acid
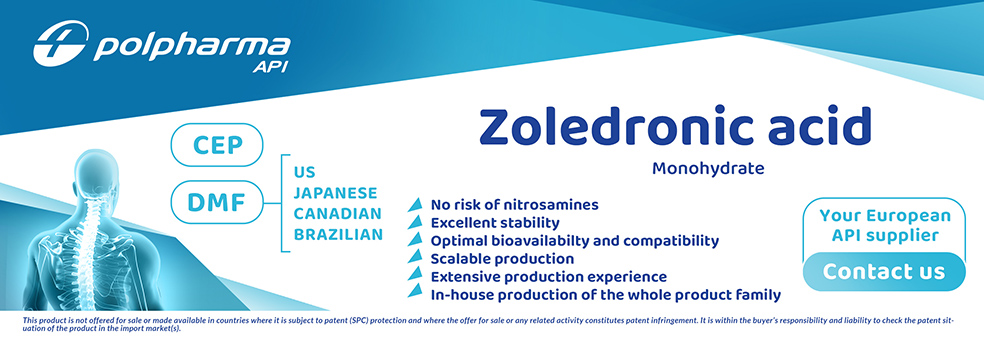
Product Description
Polpharma S.A.

-
PL
-
2015On CPHI since
-
4Certificates
-
5000+Employees
Company types
Categories
Specifications
Polpharma S.A.

-
PL
-
2015On CPHI since
-
4Certificates
-
5000+Employees
Company types
More Products from Polpharma S.A. (40)
-
Product Palbociclib
Palbocyclib
CRYSTAL FORM A
US DMF pending
DISCLAIMER
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement. It is within the buyer’s responsibility and liab... -
Product Apixaban
Apixaban
Polymorphic form N-1
> No risk of nitrosamines > Eco friendly process
> PSD flexibility via micronization
EU DMF,
US DMF,
KOREAN DMF
DISCLAIMER
Products protected by va... -
Product Empagliflozin
Empagliflozin
CRYSTALLINE ANHYDROUS
> Cryogenic process
> Green chemistry
EU DMF,
US DMF,
CHINESE DMF,
KOREAN DMF
DISCLAIMER
Products protected by valid patents... -
Product Baclofen
Baclofen
FORM B
Own innovative process
Global market leader
CEP, US
DMF,
JAPANESE DMF,
KOREAN DMF,
CANADIAN DMF
DISCLAIMER
Products protected by valid patents are not offered for s... -
Product Rivaroxaban
Rivaroxaban
MODIFICATION I
> No risk of presence of nitrosamines
> Innovative crystalization obtaining material of high purity
CEP,
US DMF,
CHINESE DMF,
KOREAN DMF
DISCLAIMER ... -
Product Trametinib
Trametinib
DMSO solvate
Oncology
OEB 5
DISCLAIMER
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement. It is within the buyer’s responsibility and liability... -
Product Piracetam
Piracetam
Form II
> Capacity and flexibility to cover high-volume demand
oral & injectable forms
CEP,
BRAZILIAN DMF"
DISCLAIMER
Products protected by valid patents are not offered for sale in cou... -
Product Linagliptin
Linagliptin
Mix A&B
> White API color
> Better impurity profile
DISCLAIMER
Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes patent infringement. It ... -
Product Elagolix sodium Amorphous form
Elagolix belongs to a class of drugs called gonadotropin-releasing hormone (GnRH) receptor antagonist. It is an oral tablet used to help treat pain caused by endometriosis .Endometriosis is a condition where tissue that should line the uterus or womb, grows outside of the uterus forming lesions. ... -
Product Pimavanserin tartrate Crystalline anhydrous form C
Pimavanserin is an antipsychotic medicine that works by changing the actions of chemicals in the brain. Pimavanserin is used to treat hallucinations and delusions caused by psychosis that is related to Parkinson's disease. Pimavanserin may also be used for purposes not listed in this medication g... -
Product Sitagliptin hydrochloride Polymorphic form III
Sitagliptin is an oral DPP-4 inhibitor used alongside diet and exercise to enhance glycemic control in individuals with type 2 diabetes mellitus. This medication works by increasing insulin and decreasing glucagon in a glucose-dependent manner, thereby improving blood sugar regulation.
P... -
Product Isavuconazole sulfate
Isavuconazonium is used to treat infections caused by certain types of fungus (aspergillosis or mucormycosis).Isavuconazonium may also be used for purposes not listed in this medication guide.
Polpharma API • Innovative manufacturing process • Chemical purity above 99,5%
Polpharma S.A. resources (5)
-
News CPHI Barcelona 2023: Tackling the Pharma Talent Precipice – Part 2
This year at CPHI Barcelona (24–26 October, 2023) we sat down with C-suite executives and HR professionals to discuss the looming talent crisis in the pharmaceutical industry. With hybrid working persisting post-pandemic and a growing skills gap, how can the pharmaceutical supply chain adjust to a changing labour force? -
Brochure API Product List 2025
Thanks to over 70 years of experience in process development, scale-up and cGMP manufacturing, we support both emerging and established pharmaceutical customers in the development and commercialization of their small molecule API clinical candidates. At our FDA-approved plant located in Central Europe, we provide end-to-end solutions from API development to scale-up allowing smooth process transfer for commercial-scale manufacturing capabilities, with world-class regulatory support. Our strong R&D management team, experienced in the development of chemical processes and complex projects, provides a wide range of solutions to our customers, applying a variety of chemical reactions and conditions, including cryogenic, High Pressure, strong base reactions, and tailor-made particle size distribution modifications. At Polpharma quality comes first. Regular FDA audits prove our reliability and credibility towards business partners around the world.
-
News KiloLab Laboratory: strategic milestone achieved!
KiloLab Laboratory – a development and production in a kilogram scale – has been launched, according to Polpharma API development strategy adopted in 2021. -
Brochure Polpharma API CDMO offer
Thanks to over 70 years of experience in process development, scale-up and cGMP manufacturing, we support both emerging and established pharmaceutical customers in the development and commercialization of their small molecule API clinical candidates. At our FDA-approved plant located in Central Europe, we provide end-to-end solutions from API development to scale-up allowing smooth process transfer for commercial-scale manufacturing capabilities, with world-class regulatory support. Our strong R&D management team, experienced in the development of chemical processes and complex projects, provides a wide range of solutions to our customers, applying a variety of chemical reactions and conditions, including cryogenic, High Pressure, strong base reactions, and tailor-made particle size distribution modifications. At Polpharma quality comes first. Regular FDA audits prove our reliability and credibility towards business partners around the world. -
Video Strategic investment HP API facility
As an EU-based Contract Development and Manufacturing Organization (CDMO) and supplier of active pharmaceutical ingredients, we are advancing our technology capabilities with an Occupational Exposure Limit (OEL) as low as 10 ng/m³ (OEB 6).
Our new capabilities enable GMP and FDA-approved kilo-scale production, offering batch sizes of up to 1.5 kg. This facility features dedicated Analytical Development Laboratories (ADL) and Process Development Laboratories (PDL) to ensure comprehensive support for all production phases. The design incorporates state-of-the-art isolator technology to ensure the safety of both personnel and products, along with industry-standard secondary containment solutions integrated into the infrastructure.
We have secured future capacity expansion through both internal enhancements and external partnerships, with the goal of doubling our production capacity.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

























.png)
.jpg)













.png)


