Why Kemwell Services ?

Product Description
Kemwell Biopharma

-
IN
-
2021On CPHI since
Kemwell Biopharma

-
IN
-
2021On CPHI since
More Products from Kemwell Biopharma (4)
-
Product Kemwell Biopharma
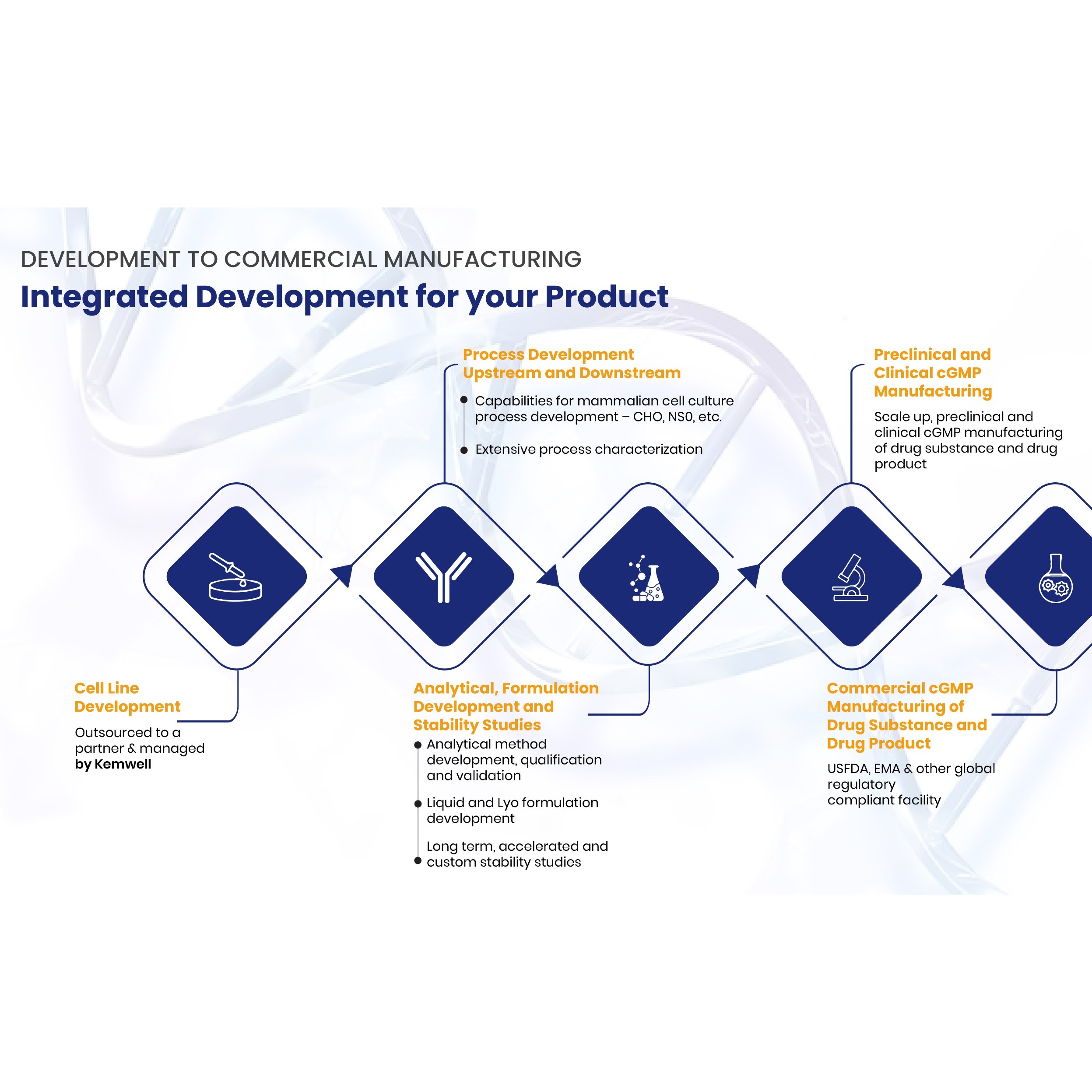
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa... -
Product Kemwell Biopharma - Analytical Development
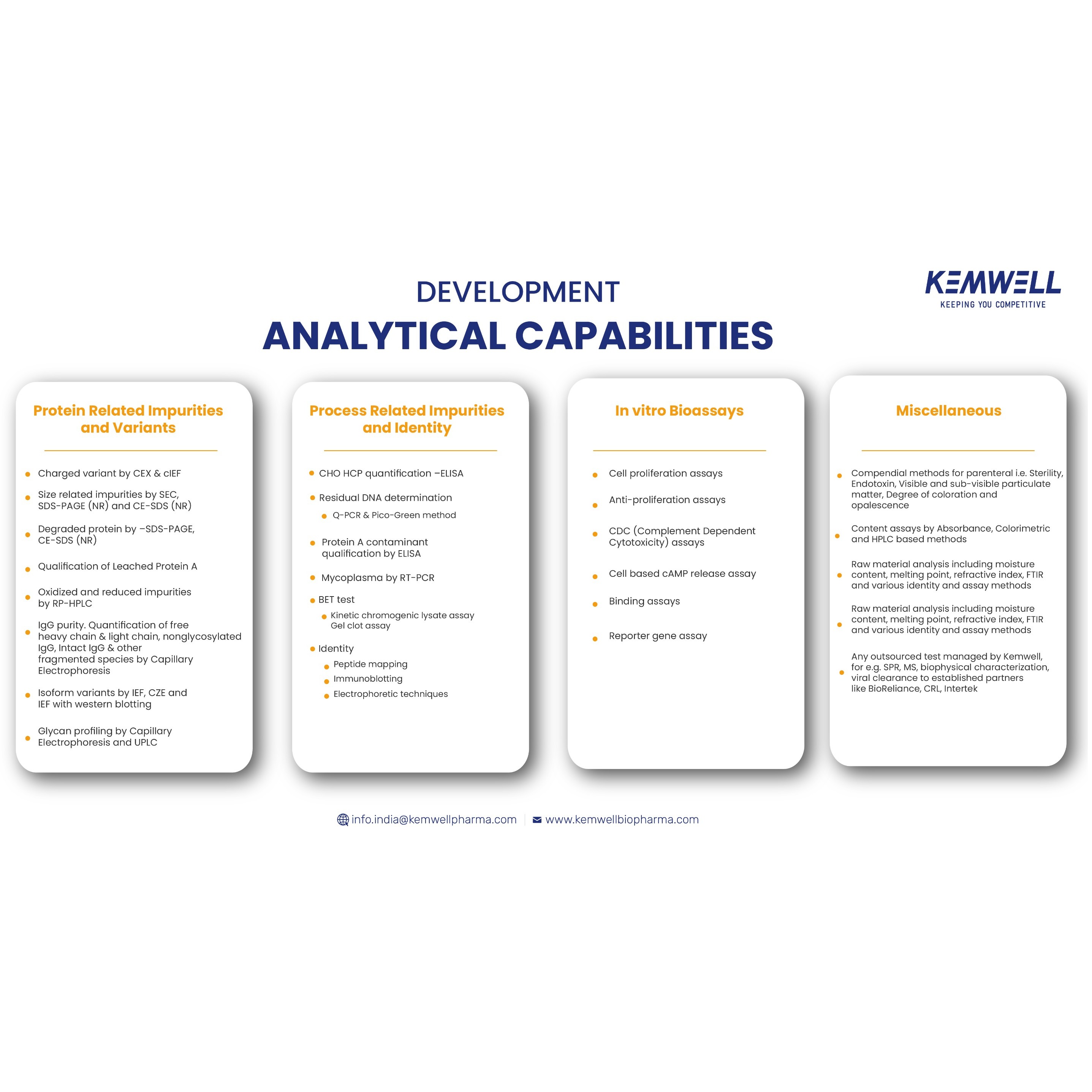
- Method development, qualification and validation services. - Platform methodologies - Offers as part of integrated or standalone service - Tests performed in-house for in-process and release, including in vitro bioassay and sterility, endotoxin. -
Product Kemwell Biopharma - Formulation Development
- Pre-formulation Studies - Formulation Screening Studies - Lyophilization Cycle Development -
Product Kemwell Biopharma - Process Development
Process Development - AMBR®250, Shake flasks, 5L, 10L (glass bioreactors), 50L (SUB), 80L (SS) – adaptable to support perfusion systems - Non-GLP and GLP tox material generation - Well characterized and geometrically similar bioreactors in pilot and GMP facility - Integrated with analytical development and...
Kemwell Biopharma resources (4)
-
News Cipla and Kemwell to form joint venture targeting respiratory biosimilars market
The agreement is expected to accelerate Cipla’s global lung leadership agenda -
Brochure Kemwell biopharma corporate Presentation
INTEGRATED SERVICE PROVIDER Drug Substance and Drug Product from same site -
Brochure Kemwell Biopharma Website link
Asia's best & largest Biologics CDMO -
Brochure Why Biopharmaceuticals can opt Kemwell Biopharma?
Kemwell provides integrated development and manufacturing services.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance