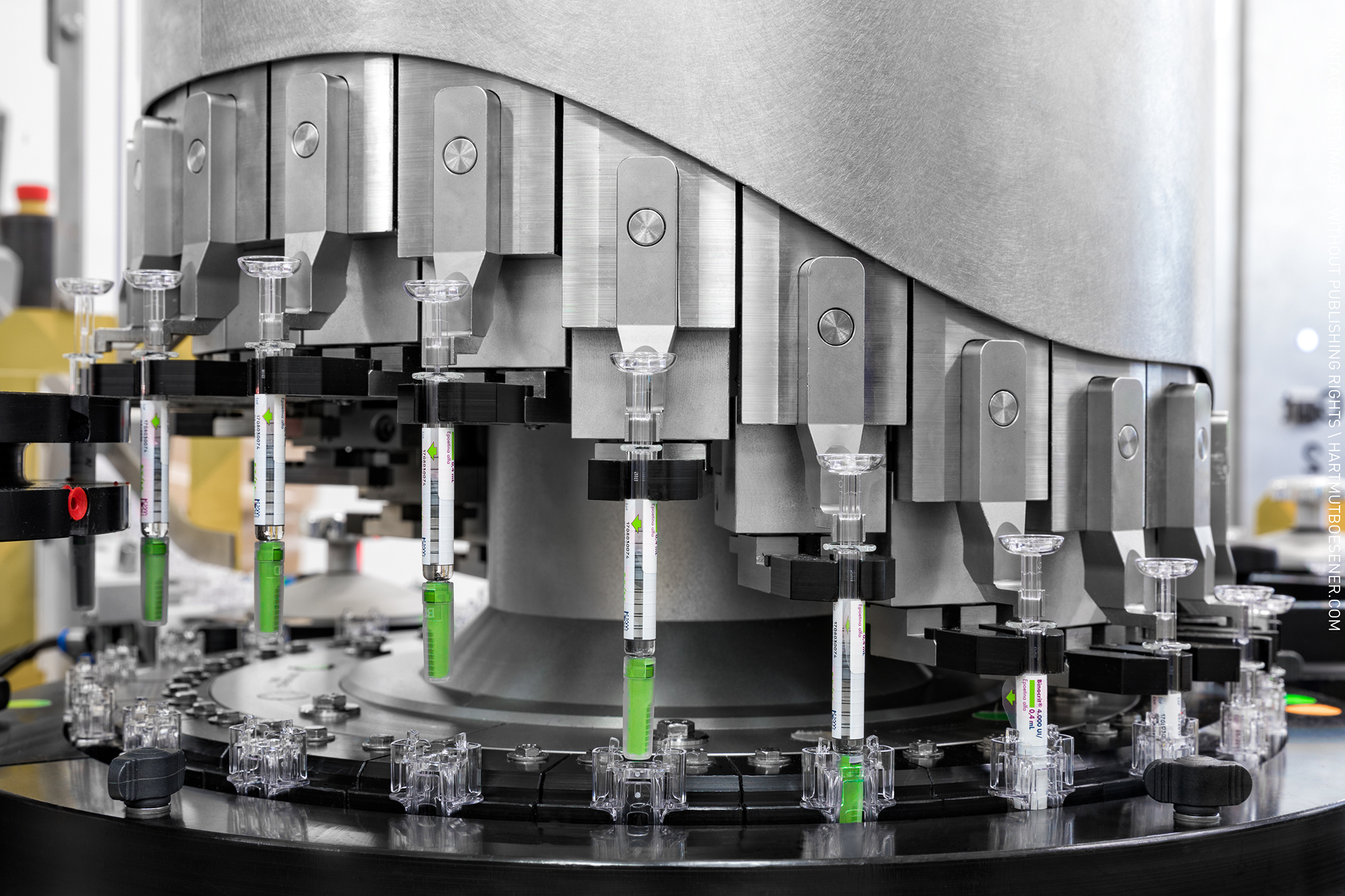
Vaccine & Biotech Fill-And Finish

Product Description
Pharma Access Pvt Ltd

-
IN
-
2017On CPHI since
Company types
Categories
Specifications
Pharma Access Pvt Ltd

-
IN
-
2017On CPHI since
Company types
More Products from Pharma Access Pvt Ltd (5)
-
Product Sterile Injectables and non injectables
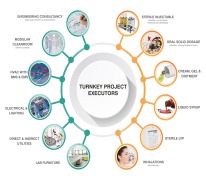
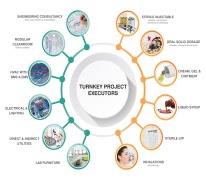
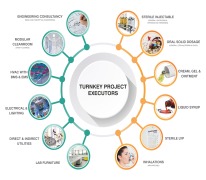
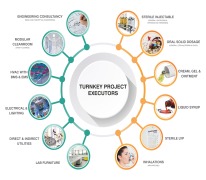
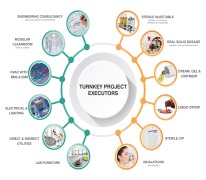
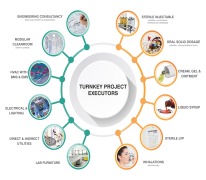
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms viz-a-viz;Inhalation (MDIs and DPIs) Sterile injectable (SVP, MVP and LVP) and non-injectables Oral Soli... -
Product Tablets and Capsules
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms and the offerings include: • Engineering Consultancy – Conceptual (In Collaboration with our EUROPEAN ... -
Product Oral Syrups - Suspension/Dry/Liquid
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms viz-a-viz;Inhalation (MDIs and DPIs) Sterile injectable (SVP, MVP and LVP) and non-injectables Oral Soli... -
Product Cream and Ointment
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms viz-a-viz;Inhalation (MDIs and DPIs) Sterile injectable (SVP, MVP and LVP) and non-injectables Oral Soli... -
Product Specialised Formulations
Pharma Access offers wide range of products which includes specialised formulations. It is processing of highly active powders with critical oel (operator exposure level), which are subject to different process steps, considering several conditions such as avoiding human contact, avoid escape of product to...
Pharma Access Pvt Ltd resources (1)
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance