URSA

Product Description
Daewoong Pharmaceutical Co.,Ltd

-
KR
-
2015On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
Categories
Daewoong Pharmaceutical Co.,Ltd

-
KR
-
2015On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
More Products from Daewoong Pharmaceutical Co.,Ltd (8)
-
Product CLOPAM(Microneedle Patch)
Microneedle is the most active and evolved form of transdermal drug delivery. Its formulation utilizes micro-sized (usually under 1mm in length) needles to deliver drugs past the stratum corneum. CLOPAM is Daewoong Therapeutics' exclusive and patented manufacturing technology that ensures efficient drug ... -
Product ENVLO®
Envlo (Enavogliflozin) is a best-in-class SGLT2 inhibitor for T2DM.
Highlights of Envlo are:
* Launched in Korea from May 2023
* Approved by Korea MFDS in 2022 for T2DM
* T2DM Phase III was completed in Korea as a monotherapy and in combinations with other anti-diabetics (du... -
Product FEXUCLUE®
Fexuclue (Fexuprazan) is the best-in-class novel anti acid secretion agent with rapid onset time and potent acid suppressive effect, addressing the growing unmet needs of PPIs
Highlights of Fexuclue are: * Launched in Korea (2022) and Philippines (2023)
* Approved in Chile and Ecuador qq... -
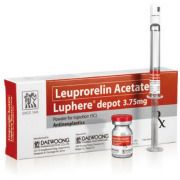
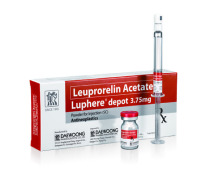
Product Luphere Depot
Luphere Depot (Leuprorelin) is a generic specialty drug of Lupron/Leuplin. Luphere Depot is a marketed product in Korea since January 2005. Currently, Luphere Depot is under development for US and Japan.
Key Features:
1) Mode of action: GnRH Agonist
2... -
Product Luphere Depot_3.75mg-Inj.
Leuprorelin Acetate
Indication: Prostate cancer, Endometriosis, Pre-menopausal breast cancer, Uterine leiomyomata (Fibroids), Central Precocious Puberty
- Luphere has two formulations; Daewoong*s proprietary patented spray-drying formulation and the emulsion formulation which will make... -
Product Olostar 20/10mg and 40/20mg - Tab.
Olmesartan medoxomil, Rosuvastatin
Indication: Concomitant Hypertension and Dyslipidemia
<World's 1st fixed dose combination of olmesartan and rosuvastatin>
1. Developed with Daewoong's patented formulation technology
2. Indicated for the treatment of both hypertensio... -
Product DWRX1010 (Colonoscopy Bowel Preparation Drug)
※ DWRXs Differentiation Points 1) Maximize compliance in the form of minitablets. 2) Containing simethicone to remove foam in the colon, enabling smooth colonoscopy. 3) Maximizes the convenience of taking medication, and low water intake. ※Development Strategy 1) Non-clinical trial (KR) and patent applica... -
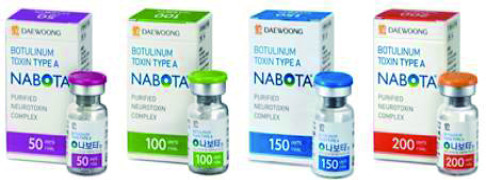
Product NABOTA®
Botulinum toxin type A
Indication: Glabella Lines (Approved in KR, US, CA, EU), Post Stroke Upper Limb Spasticity, Crow's feet, Blepharospasm, Benign Masseteric Hypertrophy (Approved in KR)
- Nabota is the only 900kDa Neurotoxin approved in the US and EU since Botox.- It has demonstra...
Daewoong Pharmaceutical Co.,Ltd resources (3)
-
Brochure Daewoong Bio Bioplant Promotion Brochure
It contains an overall introduction to Daewoong Bio's bio plant's milestones, manufacturing facilities, and production capacity. -
Video DAEWOONG PR VIDEO (EN)
We continue to take the initiative by making bold challenges and investments in R&D to leap forward as a global healthcare group. Following the 2022 launch of Fexuclue, a drug treating gastroesophageal reflux disease, the company released Envlo, a Type 2 diabetes treatment. By bolstering its R&D pipeline, Daewoong Pharmaceutical is developing first-in-class and best-in-class drugs.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance