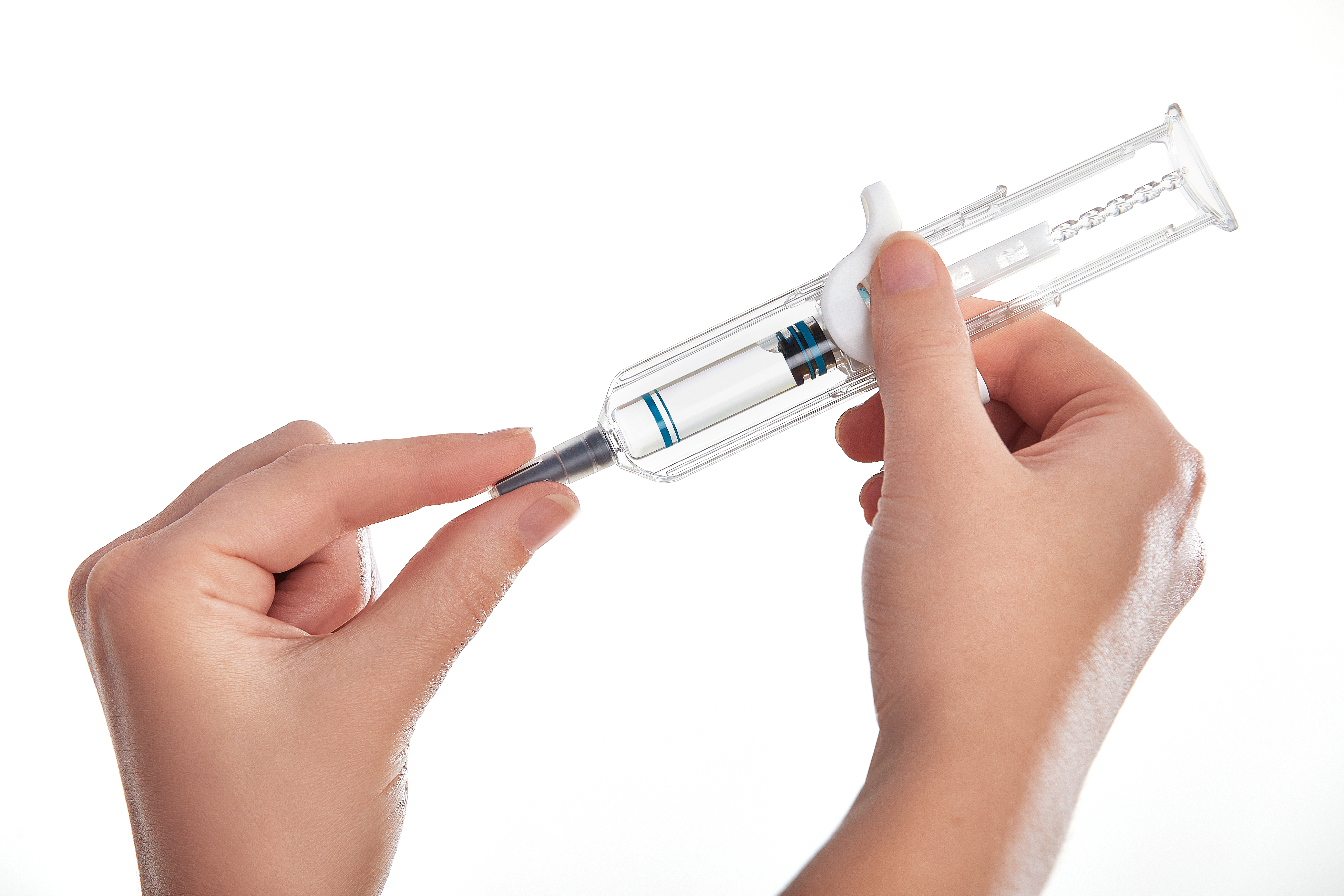
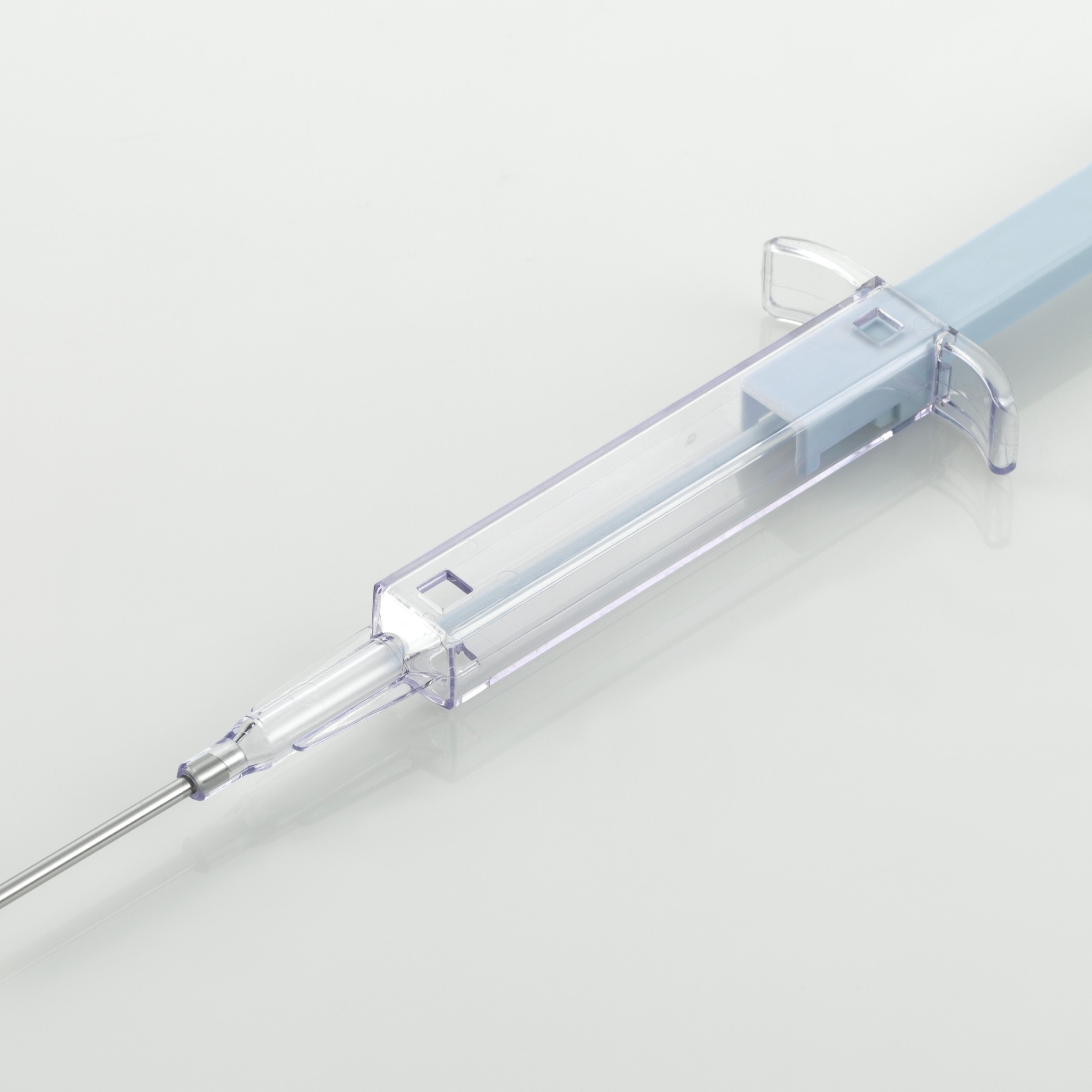
UniSafe 1 mL

Product Description
Owen Mumford Ltd

-
GB
-
2015On CPHI since
-
250 - 499Employees
Company types
Categories
Owen Mumford Ltd

-
GB
-
2015On CPHI since
-
250 - 499Employees
Company types
More Products from Owen Mumford Ltd (3)
-
Product UniSafe 2.25 mL
UniSafe® 2.25 is the latest addition to Owen Mumford Pharmaceutical Services’ UniSafe® platform. Designed for higher volume and higher viscosity drugs, UniSafe 2.25, like the on market 1 mL version, has a unique spring-free design and integrates critical safety features. UniSafe 2.25 is intuitive... -
Product Aidaptus
Aidaptus has been awarded the Red Dot award for the category Product Design 2023
Benefits
- Aidaptus® is a 2-step single use auto-injector platform, with a versatile design and intuitive drug delivery.
- Aidaptus® accommodates both 1mL and... -
Product UniSafe® Reusable Auto-injector
UniSafe® reusable connected auto-injector is a companion device for UniSafe 1mL safety syringes designed for delivery of subcutaneous medication.
The mechanical function ensures the device is always ready for use as it does not rely on batteries or chargers to deliver the medication. With a cont...
Owen Mumford Ltd resources (10)
-
News Pharmapack Europe 2023 Award Winners – Owen Mumford for Drug Delivery Innovation
We interview the winners of the Pharmapack Europe Awards 2023, which were held at Pharmapack in Paris. The winners, chosen by a jury, each developed an innovative solution in the categories of Drug Delivery Innovation, Packaging Innovation, Sustainability Initiative, Eco-Design, and Patient-Centric Design. -
Video Simplified, Smart, Sustainable Drug Delivery
In recent years there are several market trends which are influencing the design of drug delivery devices. The shift to patient care outside the traditional healthcare setting, accelerated by the pandemic, has led to an increase in patient self- treatment. This has also resulted in the need for connected drug delivery devices to enable remote patient monitoring and transfer of key medication data to healthcare providers. The data collected allows healthcare practitioners to both check levels of medication adherence and also provide the necessary interventions to help improve patient compliance. Another key influence is the growing importance of sustainability and the need to reduce the environmental impact of single-use devices.
This presentation will show how the UniSafe® reusable Auto-injector addresses these challenges by providing simplified, mechanical drug delivery with the option of added connectivity features. The reusable design also helps to reduces waste and environmental impact. -
News Pharmapack Europe Awards 2023
We are pleased to announce the winners from the Pharmapack Europe Awards 2023 at this year's event in Paris. -
Webinar Sustainable Design, Myth and Reality
Drug development, particularly of biologics can be expensive and run over several years. One of the challenges for formulation and clinical development teams is identifying devices for use in early formulation and clinical studies, where the performance characteristics and clinical safety are being assessed, without the device negatively influencing the results. Having a device platform that can be introduced into the formulation and clinical development as early as necessary with minimal impact on development timelines could go along way to simplifying the product development cycle. -
Video UniSafe – Bringing safety devices into the 21st century
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 Introduction to Owen Mumford Pharma Services, a global leader in drug delivery systems. Discussion points will be: Business Development Team Introduction Addressing future market trends for injectable drug delivery UniSafe 1 mL-a Spring-Free passive safety device for pre-filled syringes -
Webinar Optimising Drug Delivery from Clinical trial to Lifecycle Management
In this webinar, originally broadcast as part of the Pharmapack Europe show, speakers Michael Earl, Director Pharmaceutical Services, Sian Eden Business Development Manager, Olivia Houselander Business Development Manage, Denis Marteau General Manager, Pharmaceutical Services, Oli Gould Design Team Manager - Design Engineering, Research & Development, Rob Jordan Design Engineer, Research & Development from Owen Mumford discusses the introduction of auto-injectors and the technologies they originally employed some decades ago. Moving on to explore more recent trends in the market which have influenced the evolving design & development of drug delivery devices. Representatives from Owen Mumford R&D team reviews the innovative technologies employed in the Aidaptus® auto-injector and what benefits these offer to pharmaceutical companies during drug development, clinical trials and life cycle management. Our business development team will look at how elements of auto-injector design facilitate patients drug administration and ensure complete drug delivery. -
Brochure UniSafe 1mL Product Information Sheet
A spring-less, passive safety device for 1mL prefilled syringes, designed for simple assembly and use. This device has a simple 2-step final assembly, secure plunger prevents removal, spillage and repeat use.
UniSafe 1mL has the following benefits:
- User confidence with an unobscured syringe barrel for full dose visibility
- Prevention of accidental activation
- Passive needle retraction and complete shielding
- Intuitive to use - same injection technique as a prefilled syringe. -
Brochure UniSafe 2.25mL Product Information Sheet
UniSafe®️ 2.25 is the latest addition to Owen Mumford Pharmaceutical Services’ UniSafe®️ platform. Designed for higher volume and higher viscosity drugs, UniSafe 2.25, like the on market 1 mL version, has a unique spring-free design and integrates critical safety features. UniSafe 2.25 is intuitive and simple to use , its large comfortable plunger head and finger flange design make it is easy to operate, even for patients with reduced strength or dexterity.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

.jpg)




.png)



%20(1)-file145595.png)

-file128211.png)
















































.jpg)






-comp282059.jpg)