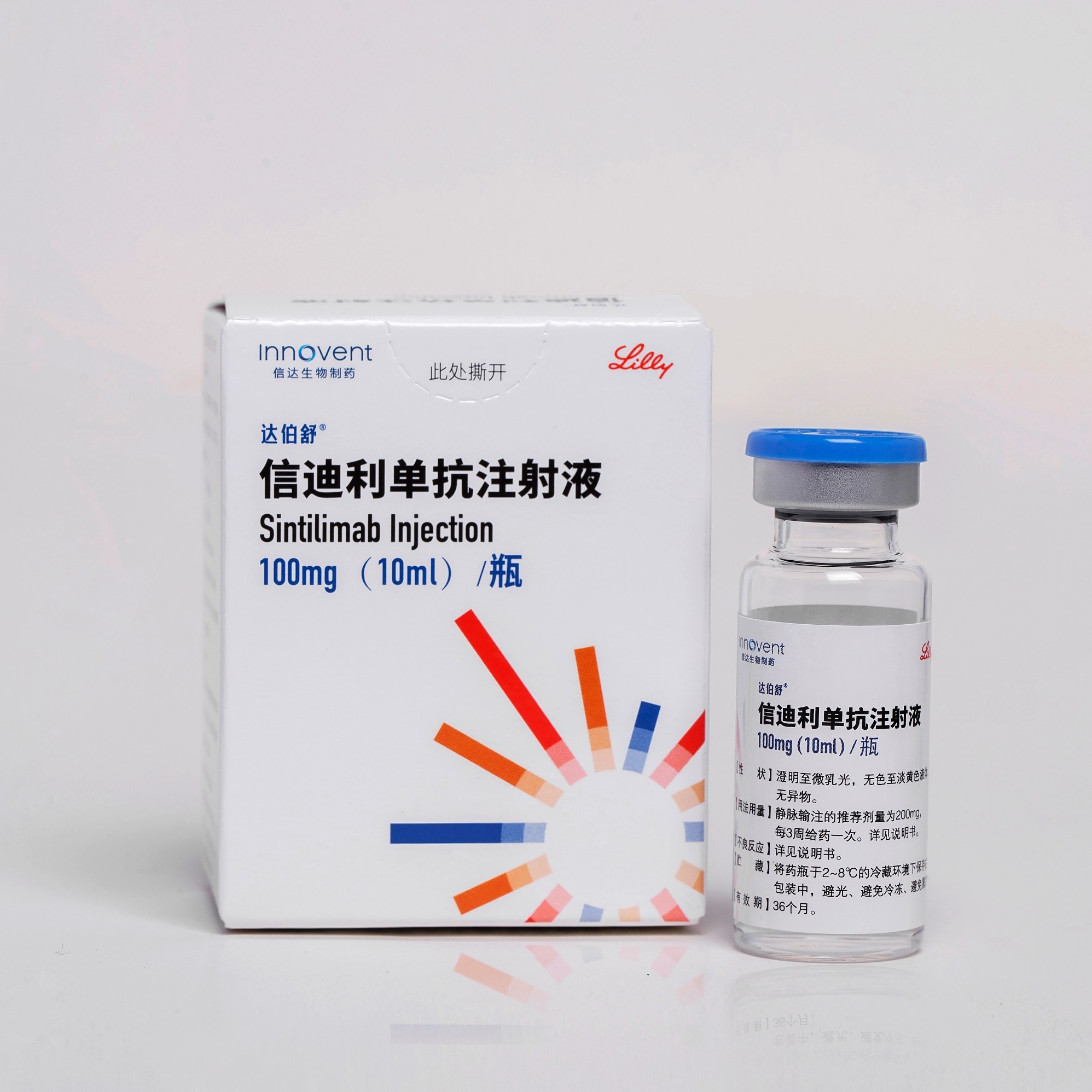
TYVYT®: Sintilimab Injection
Product Description
Altruist Biologics

-
US
-
2023On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Altruist Biologics

-
US
-
2023On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Altruist Biologics (4)
-
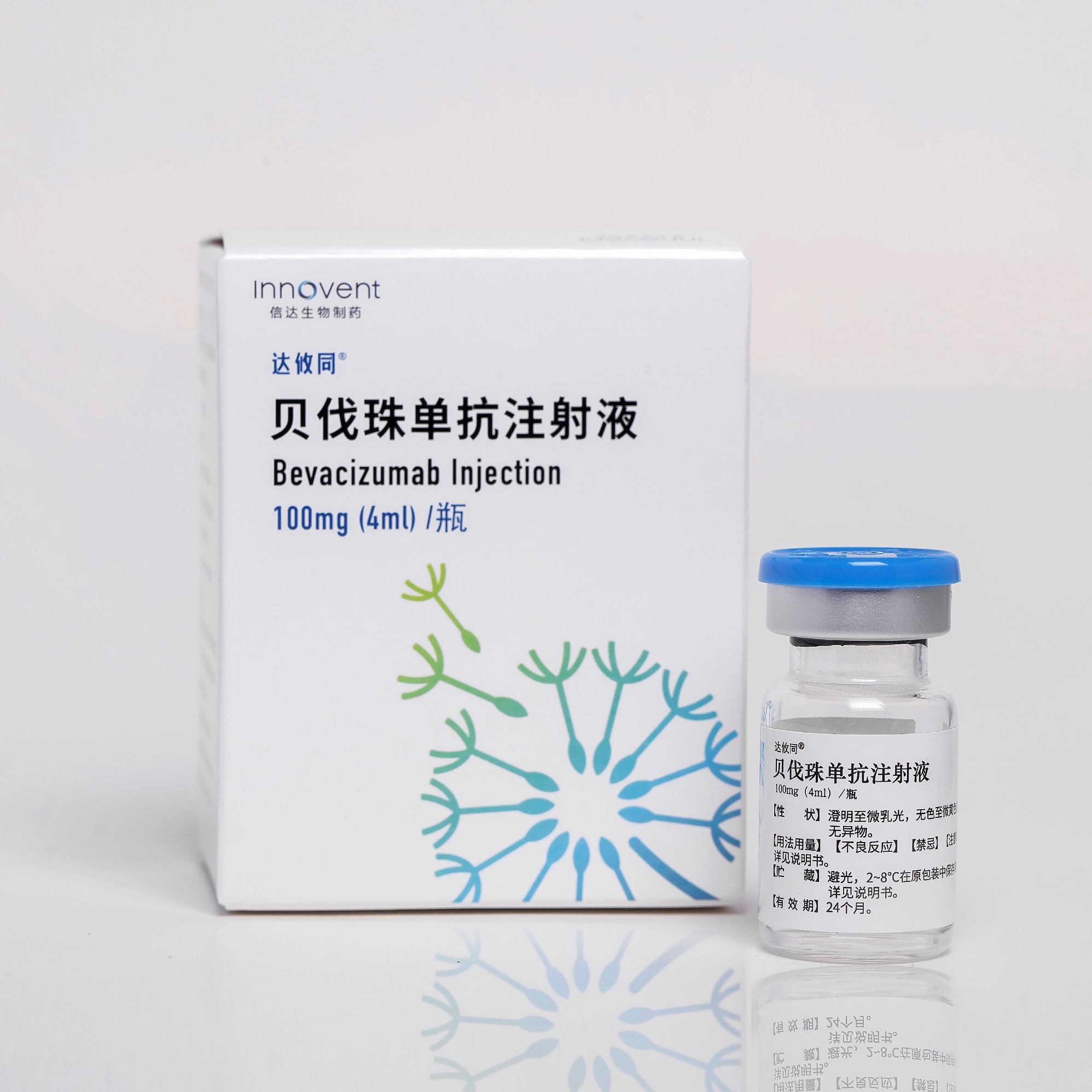
Product BYVASDA®: Bevacizumab Injection
Received NMPA marketing approval on Jun 19, 2020 Approved for advanced non-small cell lung cancer and metastatic colorectal cancer, adult recurrent glioblastoma, hepatocellular carcinoma, cervical cancer, ovarian cancer. A total of 8 indications have been approved and included in the NRDL -
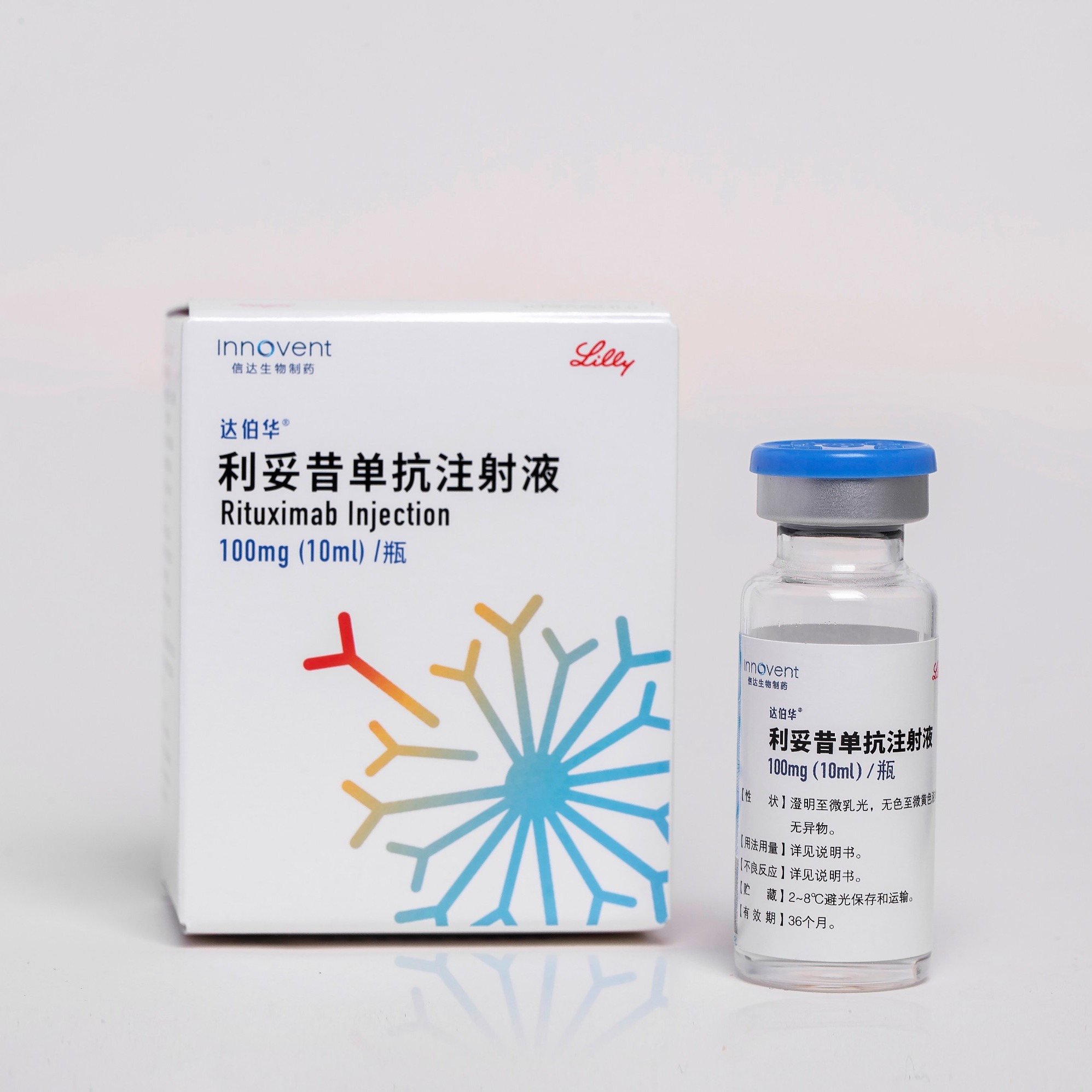
Product HALPRYZA®: Rituximab Injection
Received NMPA marketing approval on September, 2020 Approved for Diffuse large b cell lymphoma、Follicular lymphoma、Chronic lymphocytic leukemia HALPRYZA® has been approved in China for the treatment of non-Hodgkin's lymphoma and chronic lymphocytic leukemia, all of which are included in the NRDL. -
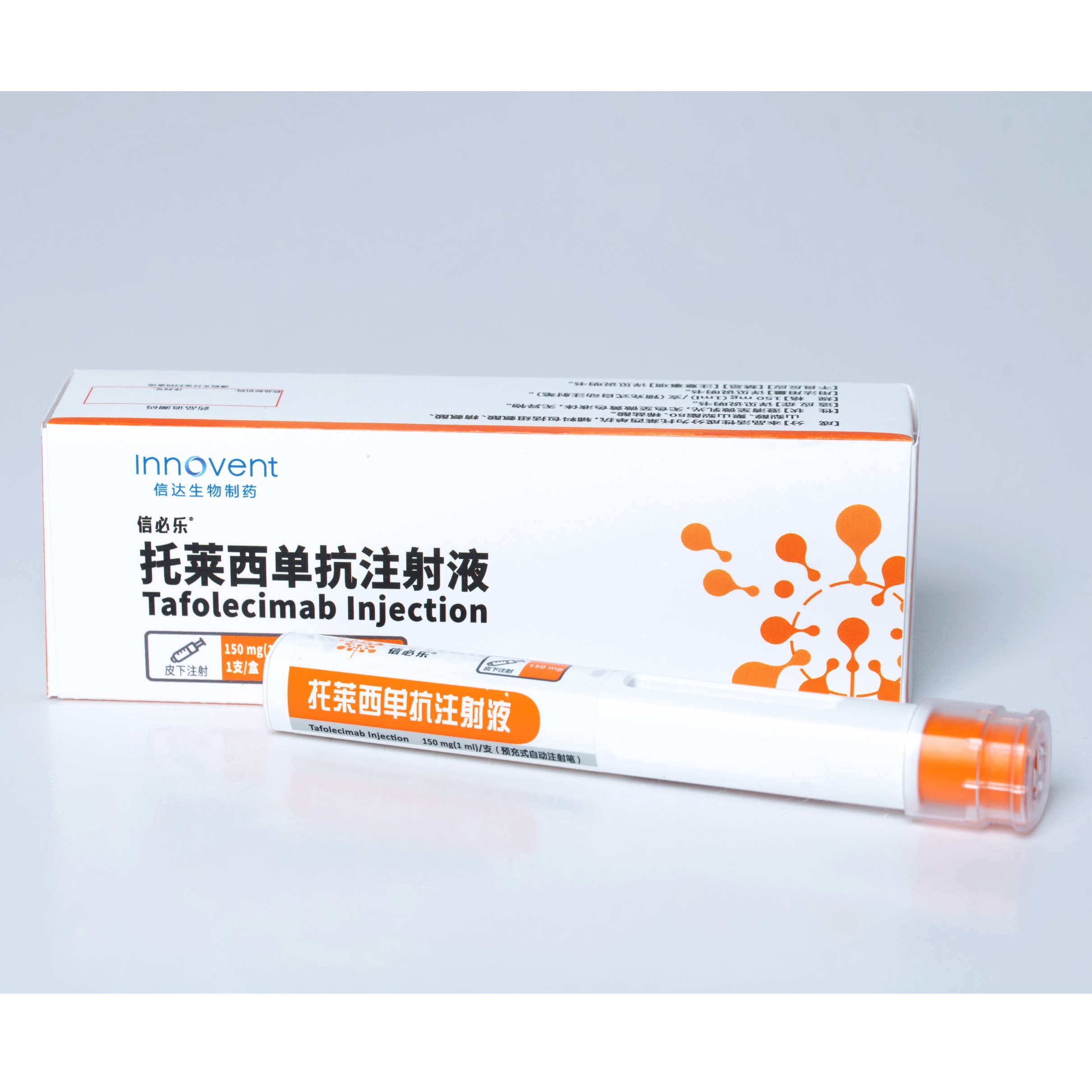
Product SINTBILO® :Tafolecimab Injection
In Aug 2023,SINTBILO® (tafolecimab injection) is approved by China's NMPA for the treatment of adult patients with primary hypercholesterolemia (including heterozygous familial and non-familial hypercholesterolemia) and mixed dyslipidemia. Tafolecimab has the advantage of a longer dosing interval compare... -
Product SULINNO®: Adalimumab Injection
Received NMPA marketing approval on August, 2020 Approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, psoriasis, and polyarticular juvenile idiopathic arthritis, pediatric plaque psoriasis, non-infectious uveitis. A total of eight approved indications have been included in the NRDL
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance